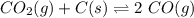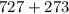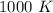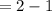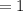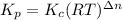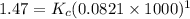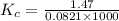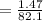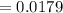Answers: 3


Another question on Chemistry


Chemistry, 22.06.2019 12:20
Consider the reaction of a(g) + b(g) + c(g) => d(g) for which the following data were obtained: experiment initial [a], mol/l initial [b], mol/l initial [c], mol/l initial rate, mol/l.s 1 0.0500 0.0500 0.0100 6.25 x 10^-3 2 0.100 0.0500 0.0100 2.50 x 10^-2 3 0.100 0.100 0.0100 1.00 x 10^-1 4 0.0500 0.0500 0.0200 6.25 x 10^-3 what is the rate law for the reaction?
Answers: 3

Chemistry, 22.06.2019 12:30
When a scientific theory has been tested and proved by the scientific community, it becomes a law
Answers: 2

Chemistry, 22.06.2019 23:30
The comparison of the number of atoms in a copper coin the size of a penny with the number of people on earth is made to illustrate which of the following? a. that atoms are indivisible b. that atoms are very small c. that atoms are very large d. that in a copper penny, there is one atom for every person on earth
Answers: 1
You know the right answer?
Kp for the reaction CO2(g) + C(s) --- 2CO(g) is 1.47 at 727°C. Calculate Kc at this temperature....
Questions

Spanish, 04.04.2021 17:50


Mathematics, 04.04.2021 17:50







Mathematics, 04.04.2021 17:50

Mathematics, 04.04.2021 17:50

English, 04.04.2021 17:50


Biology, 04.04.2021 17:50


World Languages, 04.04.2021 17:50