Chemistry, 07.03.2021 08:50 elizabethburkha
Ozone, O3, is produced in automobile exhaust by the reaction represented by the equation:
NO2 (g) + O2 (g) → NO (g) + O3 (g)
What mass of ozone is predicted to form from the reaction of 5.0 g NO2 in a car’s exhaust and excess oxygen?

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 06:30
The minerals found in bones are deposited by living cells called
Answers: 1

Chemistry, 22.06.2019 21:00
Read "who built the pyramids? ”. leave this link open while you answer the questions throughout the assignment. give at least two reasons why some people claim the pyramids of giza were constructed by aliens.
Answers: 1


Chemistry, 23.06.2019 00:30
Arrange the elements in order of increasing electronegativity. use the periodic table to you arrange the elements. p o k mg
Answers: 2
You know the right answer?
Ozone, O3, is produced in automobile exhaust by the reaction represented by the equation:
NO2 (g) +...
Questions

Mathematics, 14.12.2020 09:10





History, 14.12.2020 09:10




English, 14.12.2020 09:10

Mathematics, 14.12.2020 09:10

Mathematics, 14.12.2020 09:10


History, 14.12.2020 09:10

Advanced Placement (AP), 14.12.2020 09:10

Chemistry, 14.12.2020 09:10



English, 14.12.2020 09:10

Mathematics, 14.12.2020 09:10

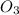 is produced.
is produced.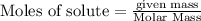
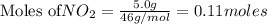
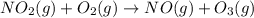
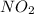 is the limiting reagent as it limits the formation of product and
is the limiting reagent as it limits the formation of product and 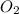 is the excess reagent.
is the excess reagent.
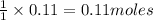 of
of