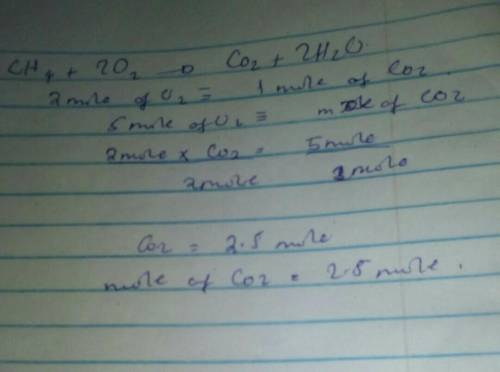The equation for the combustion of methane is
CH4+ 2O2——->CO2+2H2O
if you have 5 mol...


Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 14:00
Figure 10-1 study figure 10-1. the strong nuclear force felt by a single proton in a large nucleus
Answers: 3

Chemistry, 22.06.2019 04:30
Which is a chemical property of iron? a. it forms iron oxide (rust) when exposed to moisture and air. b. it is a gray–black metal that is hard to the touch. c. it has a melting point of 2795°f (1536°c). d. it is a good conductor of heat
Answers: 2

Chemistry, 22.06.2019 18:00
How many moles of oxygen gas are produced from the decomposition of six moles of potassium chlorate
Answers: 3

Chemistry, 22.06.2019 23:00
If two identical atoms are bonded,what kind of molecule is formed
Answers: 1
You know the right answer?
Questions

Mathematics, 08.05.2021 23:20


Chemistry, 08.05.2021 23:20


Chemistry, 08.05.2021 23:20






Mathematics, 08.05.2021 23:20





Chemistry, 08.05.2021 23:20

Social Studies, 08.05.2021 23:20