
Chemistry, 06.03.2021 01:00 hi510hello
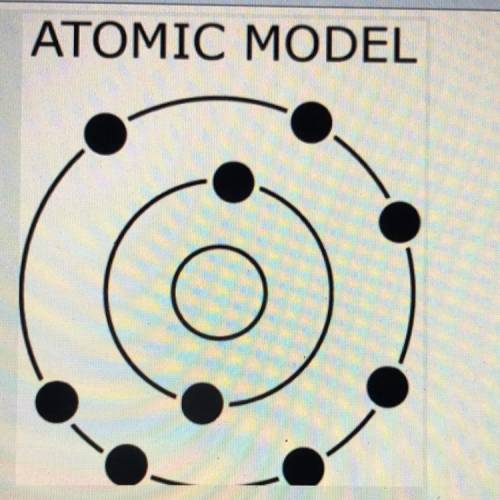
The diagram to the right shows an atomic model.
Based upon the arrangement of the electrons, which property would this atom most likely
have compared to iodine (I)?
(A)large atomic radius
(B)small nuclear charge
(C)low first ionization energy
(D)high electronegativity value

Answers: 2


Another question on Chemistry


Chemistry, 22.06.2019 20:10
The lattice enthalpy (formation of ionic solid from ions in the gas phase) for agcl(s) is -916 kj/mol and the hydration enthalpy (dissolution of gaseous ions into water) is -850 kj/mol. how much heat (in joules) is involved in forming 1l of saturated agcl solution (1.8 × 10-4 g / 100 ml water) by dissolving agcl(s)? assume solution volume does not change much upon dissolution. the equations are given below. ag+(g) + cl−(g) æ agcl(s)
Answers: 3

Chemistry, 22.06.2019 23:20
In medium-sized stars such as the sun, nuclear fusion almost always means the fusing of nuclei to form , but larger stars can produce elements as heavy as
Answers: 2

Chemistry, 23.06.2019 00:10
In as 1°, 2°, 3°, or 4°. be to . : °b: °c: °d: ° : °b: °c: °d: ° : °b: °c: °d: °e: °f: °g: °h: ° : °b: °c: °d: °e: °f: °g: °h: °i: °
Answers: 3
You know the right answer?
The diagram to the right shows an atomic model.
Based upon the arrangement of the electrons, which...
Questions

English, 11.09.2019 13:10

English, 11.09.2019 13:10




Mathematics, 11.09.2019 13:10




English, 11.09.2019 13:10


Mathematics, 11.09.2019 13:10


Social Studies, 11.09.2019 13:10


English, 11.09.2019 13:10

Business, 11.09.2019 13:10

Chemistry, 11.09.2019 13:10

World Languages, 11.09.2019 14:10



