Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 14:30
Find the mass in grams of hydrogen gas produced when 14.0 moles of hcl is added to an excess amount of magnesium.
Answers: 3


Chemistry, 22.06.2019 13:10
Select the correct answer a modure consists of glucose and water. what is the percent composition of glucose in the mixture if it contains 1.3 moles of glucose (cho total mass of the mature is 276 grams? ) and the a 1775
Answers: 1

Chemistry, 22.06.2019 17:30
Observation and experimentation have led many scientists to accept a theory about the origin of the universe. this theory is called the big bang theory. scientific evidence collected and observed by scientists around the world suggests that the universe is ever expanding from a hot and dense initial state. what makes this a scientific theory? (2 points)
Answers: 2
You know the right answer?
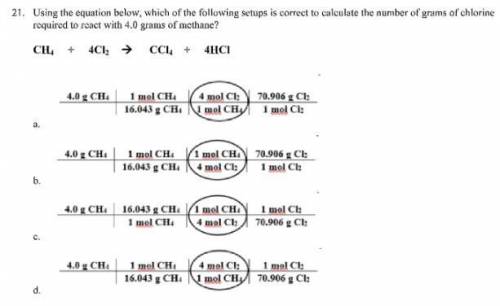
Using the equation below, which of the following setups is correct to calculate the number of grams...
Questions

Chemistry, 08.01.2021 17:40

Physics, 08.01.2021 17:40


Mathematics, 08.01.2021 17:40


English, 08.01.2021 17:40



Mathematics, 08.01.2021 17:40

Mathematics, 08.01.2021 17:40


Mathematics, 08.01.2021 17:40



Physics, 08.01.2021 17:40

Mathematics, 08.01.2021 17:40




Mathematics, 08.01.2021 17:40