
Chemistry, 06.03.2021 01:00 randyg0531
Fe2O3 + 3 CO = 2Fe + 3 CO2
In the reaction above, how many grams of Fe2O3 are required to completely react with 63.78 grams of CO?
a.121.2 g
b. 23.0 g
c 80.4 g
d. 110 g
*refer to attachment*
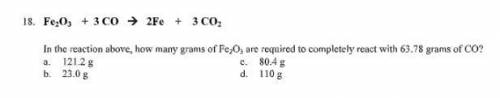

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 23:10
Nitrogen (n), phosphorus (p), and potassium (k) are the main nutrients in plant fertilizers. according to an industry convention, the numbers on the label refer to the mass percents of n, p2o5, and k2o, in that order. calculate the n: p: k ratio of a 30: 10: 10 fertilizer in terms of moles of each element, and express it as x: y: 1.0.
Answers: 1

Chemistry, 22.06.2019 04:00
Write the empirical chemical formula of calcium with a mass percent of 38.8, phosphorus with a mass percent of 20.0, and oxygen with a mass percent of 41.3.
Answers: 1

Chemistry, 22.06.2019 07:40
22. a flask containing 450 ml of 0.50 m h2so4 was accidentally knocked to the floor. how many grams of nahco, do you need to put on the spill to neutralize the acid according to the following equation: h2so4(aq)+2 nahcos(aq) na,so(aq) +2 h20()+2 co2(g) d) 38 g a) 2.3 g b) 9.5 g c) 19 g
Answers: 1

You know the right answer?
Fe2O3 + 3 CO = 2Fe + 3 CO2
In the reaction above, how many grams of Fe2O3 are required to completel...
Questions



Mathematics, 14.12.2020 21:30

Mathematics, 14.12.2020 21:30







English, 14.12.2020 21:30


Mathematics, 14.12.2020 21:30

Mathematics, 14.12.2020 21:30

Mathematics, 14.12.2020 21:30

Mathematics, 14.12.2020 21:30

Mathematics, 14.12.2020 21:30


Mathematics, 14.12.2020 21:30

Geography, 14.12.2020 21:30



