
Chemistry, 05.03.2021 23:10 tyijiapostell
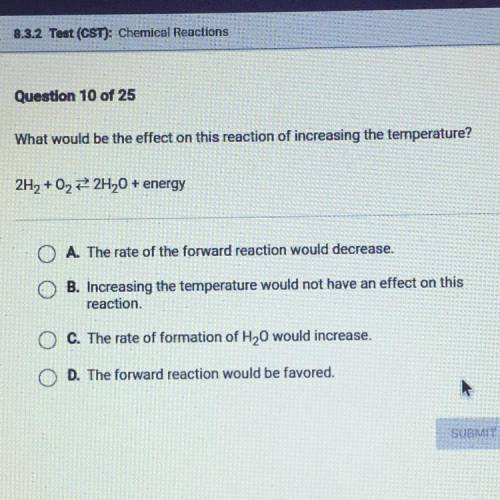
What would be the effect on this reaction of increasing the temperature?
2H + O —> 2H O + energy
2 2 2

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 13:40
Enough of a monoprotic weak acid is dissolved in water to produce a 0.0172 m solution. if the ph of the resulting solution is 2.39 at 20 °c, determine the pka for the acid.
Answers: 1

Chemistry, 22.06.2019 11:00
Problem page combustion of hydrocarbons such as pentane ( c5 h12 ) produces carbon dioxide, a "greenhouse gas." greenhouse gases in the earth's atmosphere can trap the sun's heat, raising the average temperature of the earth. for this reason there has been a great deal of international discussion about whether to regulate the production of carbon dioxide.(a) write a balanced chemical equation, including physical state symbols, for the combustion of liquid pentane into gaseous carbon dioxide and gaseous water. (b) suppose 0.350 kg of pentane are burned in air at a pressure of exactly 1 atm and a temperature of 20.0 degree c. calculate the volume of carbon dioxide gas that is produced.be sure your answer has the correct number of significant digits.
Answers: 2

Chemistry, 22.06.2019 14:30
According to le chatelier’s principle, a system in chemical equilibrium responds to stress by shifting the equilibrium in a direction that reduces the stress. normalizes the stress. increases the stress. changes the stress.
Answers: 1

You know the right answer?
What would be the effect on this reaction of increasing the temperature?
2H + O —> 2H O + energy...
Questions



History, 21.04.2021 05:00


English, 21.04.2021 05:00


Mathematics, 21.04.2021 05:00

Mathematics, 21.04.2021 05:00

Computers and Technology, 21.04.2021 05:00


Mathematics, 21.04.2021 05:00

Social Studies, 21.04.2021 05:00





Mathematics, 21.04.2021 05:00





