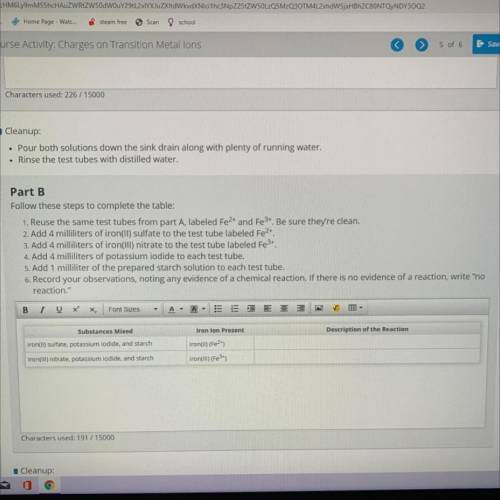
Part B
Follow these steps to complete the table:
1. Reuse the same test tubes from part A, la...

Chemistry, 05.03.2021 22:30 xxQueenPxx7432
Part B
Follow these steps to complete the table:
1. Reuse the same test tubes from part A, labeled Fe2+ and Fe3+. Be sure they're clean.
2. Add 4 milliliters of iron(II) sulfate to the test tube labeled Fe2+.
3. Add 4 milliliters of iron(III) nitrate to the test tube labeled Fe3+.
4. Add 4 milliliters of potassium iodide to each test tube.
5. Add 1 milliliter of the prepared starch solution to each test tube.
6. Record your observations, noting any evidence of a chemical reaction. If there is no evidence of a reaction, w
reaction."
B 1
U
x
x х.
Font Sizes
AA. EE
Substances Mixed
Iron lon Present
Description of the Reaction
iron(II) sulfate, potassium iodide, and starch
iron(ul) (Fe2+)
iron(III) (Fe 3+)
iron(III) nitrate, potassium iodide, and starch

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 08:30
For each of the compounds below, show that the charges on the ions add up to zero. a. kbr b. cao c. li(2)o d. cacl(2) e. alcl(3)
Answers: 2

Chemistry, 22.06.2019 09:20
How have the greenhouse gasses increased from the year 2000 to 2018
Answers: 2

Chemistry, 23.06.2019 07:00
Scuba divers use tanks of compressed air to them breathe. gases can be compressed because?
Answers: 1

You know the right answer?
Questions


Physics, 11.06.2021 07:20

Mathematics, 11.06.2021 07:20

English, 11.06.2021 07:20

Mathematics, 11.06.2021 07:20

Mathematics, 11.06.2021 07:20





Mathematics, 11.06.2021 07:20

Mathematics, 11.06.2021 07:20

Biology, 11.06.2021 07:20

Physics, 11.06.2021 07:20


Mathematics, 11.06.2021 07:20



Law, 11.06.2021 07:20



