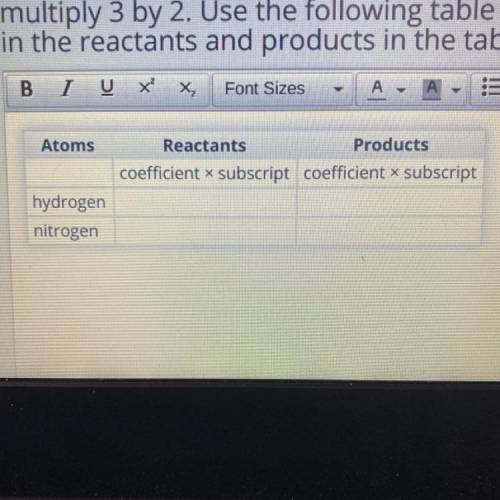
N2 + 3H2 2NH3

Chemistry, 05.03.2021 19:40 kayliebug2003
(image is the layout of the table)
Let's look at the chemical equation again:
N2 + 3H2 2NH3
This time, you'll use a different method to count the atoms. Multiply the coefficient by the
subscript for each atom. For example, to find the number of hydrogen atoms in 3H2,
multiply 3 by 2. Use the following table to organize your work. Fill in the number of atoms
in the reactants and products in the table.
Atoms
Reactants
Products
coefficient x subscript coefficient x subscript
hydrogen
nitrogen

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 20:30
An exothermic reaction is conducted in an insulated calorimeter filled with water. the calorimeter is then sealed so that there is no heat exchanged between the contents of the container and the surrounding air. which of the following statements is true about the reaction?
Answers: 1

Chemistry, 22.06.2019 01:30
What is the value of keq for the reaction expressed in scientific notation
Answers: 1

Chemistry, 22.06.2019 14:00
How is the atomic number of a nucleus changed by alpha decay
Answers: 2

Chemistry, 22.06.2019 14:00
Calculate the frequency of a wave in a spring toy. the wave has a speed of 1.1 meters per second and a wavelength of 0.1 meters. *
Answers: 2
You know the right answer?
(image is the layout of the table)
Let's look at the chemical equation again:
N2 + 3H2 2NH3
N2 + 3H2 2NH3
Questions


Mathematics, 22.12.2020 17:40


Mathematics, 22.12.2020 17:40

Chemistry, 22.12.2020 17:40


English, 22.12.2020 17:40


Mathematics, 22.12.2020 17:40


Mathematics, 22.12.2020 17:40




Mathematics, 22.12.2020 17:40


History, 22.12.2020 17:40


Social Studies, 22.12.2020 17:40

Mathematics, 22.12.2020 17:40



