Answers: 3


Another question on Chemistry



Chemistry, 22.06.2019 10:30
Aglow stick contains a glass vial with chemicals. when the glow stick is bent, the vial breaks and the chemicals react to produce a glow. a science student observes that a glow stick kept in the freezer glows for a longer duration than a glow stick kept at room temperature. what conclusion can be drawn based on the observation? be sure to note the outcome and test variables in the conclusion.
Answers: 1

Chemistry, 22.06.2019 11:00
Imagine that twenty i.u.’s of enzyme z were catalyzing the above reaction for one minute, under vmaxconditions, in a 3.00 ml assay volume. the assay is buffered with 20 mm phosphate buffer, ph 7.60. what will the ph be at the end of that one minute?
Answers: 2
You know the right answer?
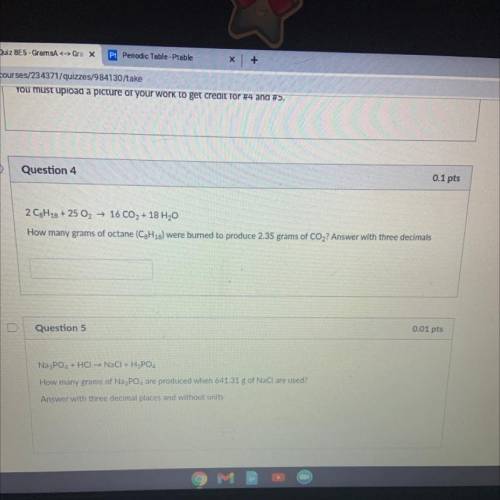
2 C8H18 + 25 O2 → 16 CO2 + 18 H20
How many grams of octane (C3H18) were burned to produce 2.35 gram...
Questions

Arts, 30.11.2020 23:50






Mathematics, 30.11.2020 23:50

Mathematics, 30.11.2020 23:50




Social Studies, 30.11.2020 23:50

Mathematics, 30.11.2020 23:50

Mathematics, 30.11.2020 23:50

Biology, 30.11.2020 23:50

Mathematics, 30.11.2020 23:50


Mathematics, 01.12.2020 01:00