
Chemistry, 05.03.2021 19:10 josephgperez
I’ll give brainliest 1. Use the balance and the 30-ml beaker places to measure 19 g of table sugar. To do this, place the beaker on the balance, the zero the balance. Add sugar to the beaker until the balance reads 19g. Mix the sugar with 50 mL of distilled water in the 150 mL beaker, stir until dissolved.
2. Prepare an ice bath in clear cup by filling it nearly full of ice. Put a small amount of cold water into the cup and then add about 1 tablespoon of table salt over the ice. Stir this with the tablespoon and measure the temperature. While stirring, continue to add table salt to the cup until the temperature is -15°C or lower.
3. Fill a test tube about 2/3 full with the sucrose solution.
4. Places the thermometer in the test tube. Hold the thermometer and the test tube together so the thermometer stays in the place in test tube and place this in the ice bath. Be careful not to get any salt water from the cup into the test tube.
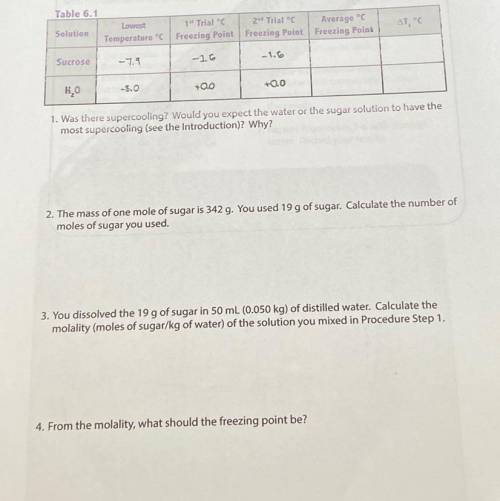
5. While holding the test tube thermometer assembly, observe for the formation of ice crystals. The temperature when ice crystals first form may be below the normal freezing temperature (The solution may become supercooled). Record the lowest observed temperature just as the ice crystals first form. Remove the test tube thermometer assembly from the ice bath. Wait for most of the ice to melt. This will be the freezing point. Record the temperature in table 6.1.
6. Let the remainder of the ice in the test tube melt completely. Repeat procedures 4-6 once more and record the results.
7. Repeat procedures 3-6 with distilled water. Record your results

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 13:00
Metallic bonds are good conductors of electricity true or false
Answers: 2

Chemistry, 22.06.2019 08:00
Match the mixture with the substance// i really need on this guys (it’s a pic btw)
Answers: 1

Chemistry, 22.06.2019 12:30
If 22.5 liters of oxygen reacted with excess of hydrogen, how many liters of water vapor could be produced?
Answers: 3

Chemistry, 22.06.2019 13:00
What happens to the average kinetic energy of a gas when the particles of the gas collide against each other at a constant temperature and volume? explain your answer.
Answers: 3
You know the right answer?
I’ll give brainliest 1. Use the balance and the 30-ml beaker places to measure 19 g of table sugar....
Questions




Mathematics, 17.11.2020 20:30

Mathematics, 17.11.2020 20:30


Mathematics, 17.11.2020 20:30

History, 17.11.2020 20:30


Mathematics, 17.11.2020 20:30

History, 17.11.2020 20:30

Mathematics, 17.11.2020 20:30

Mathematics, 17.11.2020 20:30

History, 17.11.2020 20:30

Health, 17.11.2020 20:30



History, 17.11.2020 20:30

Mathematics, 17.11.2020 20:30



