
Chemistry, 05.03.2021 19:00 sydneepollitt32
Carbon dioxide and water react to form methanol and oxygen, like this:
2CO2(g) + 4H2O(g) → 2CH3OH(l) + 3O2 (g)
At a certain temperature, a chemist finds that a 7.5L reaction vessel containing a mixture of carbon dioxide, water, methanol, and oxygen at equilibrium has the following composition:
Compound Amount
CO2 3.28g
H2O 3.86g
CH3OH 1.51g
O2 2.80g
Required:
Calculate the value of the equilibrium constant Kc for this reaction. Round your answer to 2 significant digits.

Answers: 1


Another question on Chemistry


Chemistry, 22.06.2019 14:50
Which of the following is most likely true about water in chemical systems? a) water dissolves nonpolar ionic compounds. b) water dissociates ionic compounds. c) water dissociates covalent molecules. d) water dissolves nonpolar covalent substances.
Answers: 1

Chemistry, 22.06.2019 15:00
Answer explain why it is not possible to deduce a complete order of reactivity.
Answers: 3

Chemistry, 22.06.2019 20:00
Listenbase your answer to the question on the information below.nuclear radiation is harmful to living cells, particularly to fast-growing cells, such as cancer cells and blood cells. an external beam of the radiation emitted from a radioisotope can be directed on a small area of a person to destroy cancer cells within the body.cobalt-60 is an artificially produced radioisotope that emits gamma rays and beta particles. one hospital keeps a 100.0-gram sample of cobalt-60 in an appropriate, secure storage container for future cancer treatment.which choice represents the correct product for the beta decay of the co-60? fe-60ni-60fe-61ni-61
Answers: 2
You know the right answer?
Carbon dioxide and water react to form methanol and oxygen, like this:
2CO2(g) + 4H2O(g) → 2CH3OH(l...
Questions






Mathematics, 07.04.2020 15:48


Mathematics, 07.04.2020 15:48


Computers and Technology, 07.04.2020 15:48










![\frac{[O_2]^3}{[CO_2]^2[H_2O]^4}](/tpl/images/1172/1518/03065.png)
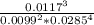 = 24x10³
= 24x10³

