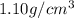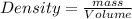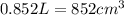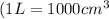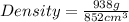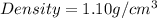A chemist working as a safety inspector finds an unmarked bottle in a lab cabinet. A note on the door of the cabinet says the cabinet is used to store bottles of carbon tetrachloride, diethylamine, methyl acetate, ethanolamine, and dimethyl sulfoxide.
The chemist plans to try to identify the unknown liquid by measuring the density and comparing to known densities. First, from his collection of Material Safety Data Sheets (MSDS), the chemist finds the following information:
Liquid Density
Tetrahydrofuran 0.89·gcm^−3
Carbon tetrachloride 1.6·gcm^−3
Pentane 0.63·gcm^−3
Dimethyl sulfoxide 1.1·gcm^−3
Acetone 0.79·gcm^−3.
Next, the chemist measures the volume of the unknown liquid as 0.852L and the mass of the unknown liquid as 938.g . Calculate the density of the liquid.

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 10:20
Gwhich r group would most likely be found in a hydrophobic area of the tertiary structure of a globular protein? which r group would most likely be found in a hydrophobic area of the tertiary structure of a globular protein? −ch2−oh −ch2−o||c−nh2 −ch2−coo− −ch2−ch2−ch2−ch2−n+h3
Answers: 3



Chemistry, 23.06.2019 03:00
Asample of sea water contains 6.28g of sodium chloride per litre of solution. how many milligrams of sodium chloride would be contained in 15.0ml of this solution?
Answers: 3
You know the right answer?
A chemist working as a safety inspector finds an unmarked bottle in a lab cabinet. A note on the doo...
Questions

Mathematics, 13.07.2019 12:30

Mathematics, 13.07.2019 12:30

Computers and Technology, 13.07.2019 12:30

Mathematics, 13.07.2019 12:30

Biology, 13.07.2019 12:30

History, 13.07.2019 12:30

English, 13.07.2019 12:30


Mathematics, 13.07.2019 12:30

History, 13.07.2019 12:30






History, 13.07.2019 12:30

History, 13.07.2019 12:30



Mathematics, 13.07.2019 12:30