
Chemistry, 05.03.2021 06:50 rleiphart1
When 1.50 mol CO2 and 1.50 mol H2 are placed in a 3.00-Lcontainer at 395 °C, the following reaction
CO2(g) + H2(g) = CO(g) + H2O(g). If Kc = 0.802,
what are the concentrations of each substance in the equilibrium mixture?
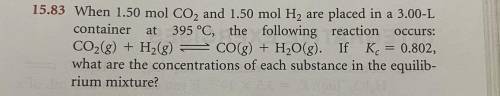

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 03:00
Match term definition ellipse a) diagonal cross section of a cylinder circle b) diagonal cross section through the widest part of a sphere sphere c) cross section parallel to the base of a cone great circle d) shape created when a semi-circle is rotated around the y-axis triangle e) perpendicular cross section of a cone
Answers: 1

Chemistry, 22.06.2019 07:50
In which situation can a mixture always be called a solution
Answers: 3

Chemistry, 22.06.2019 10:30
Use this information to determine the number of calends electrons in the atoms. which of the following correctly compares the stability of the two atoms? a) both are unreactive b) both are highly reactive c) a is unreactive and d is reactive d) a is reactive and d is unreactive
Answers: 2

Chemistry, 22.06.2019 15:30
What best discribes the relationship between wavelength and frequency in a electromagnetic wave
Answers: 1
You know the right answer?
When 1.50 mol CO2 and 1.50 mol H2 are placed in a 3.00-Lcontainer at 395 °C, the following reaction...
Questions


Mathematics, 01.07.2021 16:50





Business, 01.07.2021 16:50

Mathematics, 01.07.2021 16:50

Mathematics, 01.07.2021 16:50


English, 01.07.2021 16:50


History, 01.07.2021 16:50

English, 01.07.2021 16:50

Social Studies, 01.07.2021 16:50

English, 01.07.2021 16:50

Mathematics, 01.07.2021 16:50



Chemistry, 01.07.2021 16:50



