
Chemistry, 05.03.2021 04:00 ariloveshorses
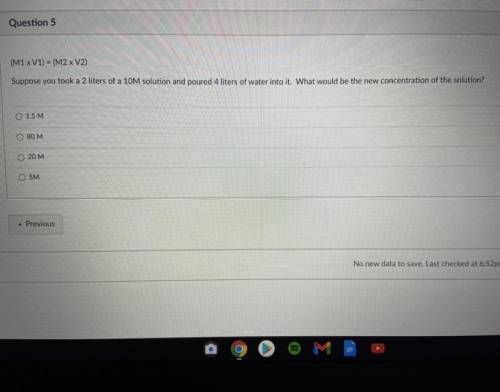
Suppose you a 2 of 10M solution and poured 4 liters of water into it. What would be the new concentration of the solution?

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 22:20
One or more substances changing into one or more substances is an example of a
Answers: 1

Chemistry, 22.06.2019 06:00
Compare and contrast physical changes with chemical changes.
Answers: 3

Chemistry, 22.06.2019 14:50
Consider the following multistep reaction: a b→ab(slow) a ab→a2b(fast)−−−−−−−−−−−−−−−−− 2a b→a2b(overall) based on this mechanism, determine the rate law for the overall reaction. express your answer in standard masteringchemistry format. for example, if the rate law is k[a]3[b]2 type k*[a]^3*[b]^2
Answers: 3

Chemistry, 22.06.2019 19:00
A4.86 g piece of metal was placed in a graduated cylinder containing 15.5 ml of water. the water level rose to 17.3 ml. what is the density of the metal. i need the steps of how to solve it to so i can use a formula to work out other problems.
Answers: 1
You know the right answer?
Suppose you a 2 of 10M solution and poured 4 liters of water into it. What would be the new concentr...
Questions



Mathematics, 04.02.2021 02:20


Business, 04.02.2021 02:20

Social Studies, 04.02.2021 02:20

English, 04.02.2021 02:20

English, 04.02.2021 02:20

Mathematics, 04.02.2021 02:20


Chemistry, 04.02.2021 02:20


History, 04.02.2021 02:20

Biology, 04.02.2021 02:20

Mathematics, 04.02.2021 02:20



Mathematics, 04.02.2021 02:20


Social Studies, 04.02.2021 02:20



