
Chemistry, 04.03.2021 23:40 jasjoh39p0rp6i
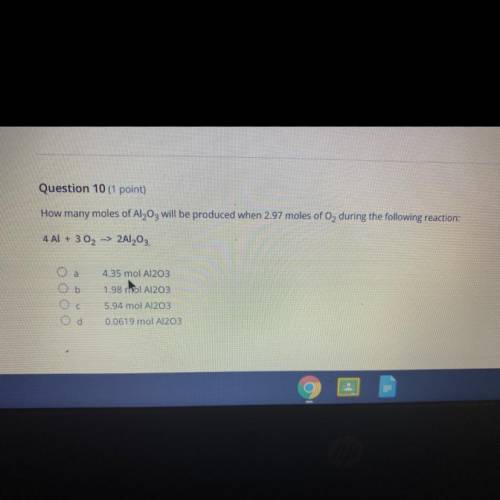
Question 10 (1 point)
How many moles of Al2O3 will be produced when 2.97 moles of Oz during the following reaction:
4 Al + 302 --> 2Al2O3.
а
4.35 mol A1203
Ob
1.98 ml Al2O3
Oc
5.94 mol A1203
d
0.0619 mol Al2O3

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 11:00
Freezing and boiling are endothermic processes. this means that these processes absorb energy from their surroundings in order to occur. use this information and the data you collected in the phase change gizmo to describe what happens to the temperature of water when you boil it, then explain why this result occurs.
Answers: 1


Chemistry, 22.06.2019 19:10
Δu of , in kj/kg, as it isto k, (a)as a of , (b) at , (c) at .
Answers: 2

Chemistry, 23.06.2019 02:00
Which statement is true about the model of the electromagnetic spectrum a: it change the frequencies of light. b: it compare wavelengths of light. c: the color of light waves can be changed using the model. d: the intensities of light waves can be decreased using the model.
Answers: 2
You know the right answer?
Question 10 (1 point)
How many moles of Al2O3 will be produced when 2.97 moles of Oz during the fol...
Questions



Chemistry, 27.03.2020 22:34





Mathematics, 27.03.2020 22:34



Mathematics, 27.03.2020 22:34

English, 27.03.2020 22:34

Mathematics, 27.03.2020 22:34

History, 27.03.2020 22:34


Mathematics, 27.03.2020 22:34

Mathematics, 27.03.2020 22:34



Mathematics, 27.03.2020 22:34



