Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 16:30
What is the force of attraction between the particles in a salt crystal
Answers: 2

Chemistry, 22.06.2019 19:30
What is the common name for the compound shown here? enter the common name of the compound shown?
Answers: 2

Chemistry, 22.06.2019 21:30
Athe top of a hill, an athlete on a skateboard has x joules of mechanical energy. how much mechanical energy will she have at the bottom of the hill? ignore the effects of friction.
Answers: 1

Chemistry, 22.06.2019 22:00
Choose all the answers that apply. fluorine (f) has an atomic number of 9 and an atomic weight of 18.99. fluorine has a. 9 protons b. 10 neutrons c. 18 electrons d. an atomic mass of 19 e. at least one isotope
Answers: 1
You know the right answer?
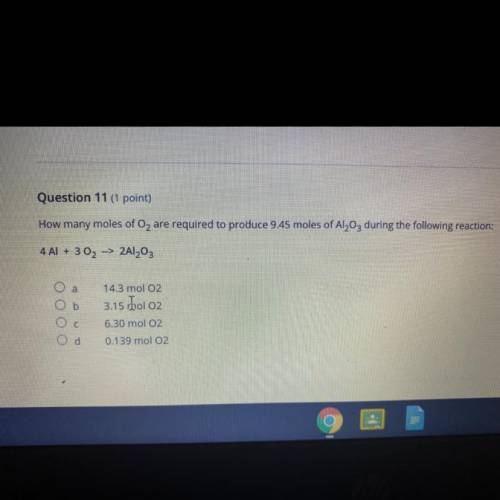
Question 11 (1 point)
How many moles of O2 are required to produce 9.45 moles of Al2O3 during the f...
Questions

Geography, 17.09.2020 14:01

Geography, 17.09.2020 14:01

Mathematics, 17.09.2020 14:01

Mathematics, 17.09.2020 14:01

Social Studies, 17.09.2020 14:01

Mathematics, 17.09.2020 14:01

Physics, 17.09.2020 14:01

Mathematics, 17.09.2020 14:01

Mathematics, 17.09.2020 14:01

Mathematics, 17.09.2020 14:01

Mathematics, 17.09.2020 14:01

English, 17.09.2020 14:01

Mathematics, 17.09.2020 14:01

Mathematics, 17.09.2020 14:01

Mathematics, 17.09.2020 14:01

Mathematics, 17.09.2020 14:01

Biology, 17.09.2020 14:01

Mathematics, 17.09.2020 14:01

Mathematics, 17.09.2020 14:01

Mathematics, 17.09.2020 14:01