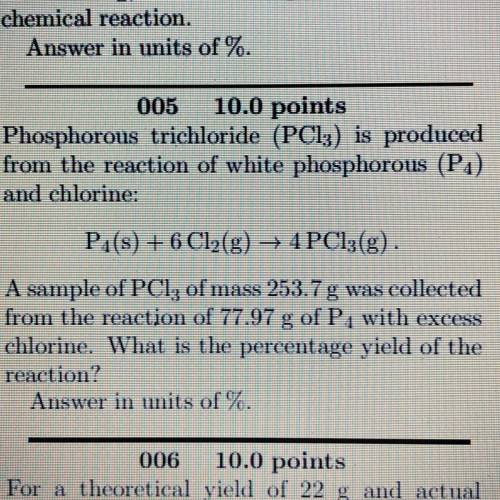
Phosphorous trichloride (PC13) is produced
from the reaction of white phosphorous (P1)
and ch...

Chemistry, 04.03.2021 22:10 Jamilia561
Phosphorous trichloride (PC13) is produced
from the reaction of white phosphorous (P1)
and chlorine:
Pa(s) + 6 Cl2(g) → 4PC13(g)
A sample of PCl, of mass 253.7 g was collected
from the reaction of 77.97 of P, with excess
chlorine. What is the percentage yield of the
reaction?
Answer in units of %

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 18:00
Aballoon inflated with three breaths of air has a volume of 1.7 l. at the same temperature and pressure, what is the volume of the balloon if five more same-sized breaths are added to the balloon?
Answers: 3


Chemistry, 22.06.2019 17:00
Which statement is true about a catalyst? a: a catalyst decreases the rate of the reaction. b. a catalyst is consumed during a chemical reaction. c. a catalyst lowers the activation energy of a reaction. d. a catalyst increases the reactant concentration during a reaction.
Answers: 1

Chemistry, 23.06.2019 02:00
Which best describes the present-day universe? opaque, expanding very slowly, stars produce heavy elements transparent, expanding at an accelerated rate, stars produce heavy elements opaque, expanding at an accelerated rate, stars produce only hydrogen and helium transparent, expanding very slowly, stars produce only hydrogen and helium
Answers: 1
You know the right answer?
Questions




Geography, 24.02.2021 05:20

Mathematics, 24.02.2021 05:20

Social Studies, 24.02.2021 05:20


Mathematics, 24.02.2021 05:20


English, 24.02.2021 05:20

Health, 24.02.2021 05:20

Mathematics, 24.02.2021 05:20






World Languages, 24.02.2021 05:20




