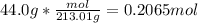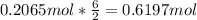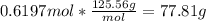Chemistry, 04.03.2021 16:00 tasnimsas3
6CuNO3 + Al2(SO4)3 → 3Cu2SO4 + 2Al(NO3)3
Molar mass of CuNO3 125.56 g/mol
Molar mass of Al(NO3)3 213.01 g/mol
How many grams of copper (I) nitrate (CuNO3) are required to produce 44.0 grams of aluminum nitrate (Al(NO3)3)?

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 02:30
List four observations that indicate that a chemical reaction may be taking place
Answers: 1

Chemistry, 22.06.2019 06:00
Compare and contrast physical changes with chemical changes.
Answers: 1

Chemistry, 22.06.2019 11:20
Which of the following contributes to the structural rigidity of cellulose? adjacent glucose polymers are stabilized by hydrogen bonding. glucose residues are joined by (α1→4) linkages. cellulose is a highly branched molecule. the conformation of the glucose polymer is a coiled structure.
Answers: 2

Chemistry, 22.06.2019 12:30
Sodium sulfate dissolves as follows: na2so4(s) → 2na+(aq) + so42- (aq). how many moles of na2so4 are required to make 1.0 l of solution in which the na concentration is 0.10 m?
Answers: 2
You know the right answer?
6CuNO3 + Al2(SO4)3 → 3Cu2SO4 + 2Al(NO3)3
Molar mass of CuNO3 125.56 g/mol
Molar mass of Al(NO...
Molar mass of Al(NO...
Questions

English, 08.12.2021 01:00

Mathematics, 08.12.2021 01:00

Mathematics, 08.12.2021 01:00




Mathematics, 08.12.2021 01:00

Mathematics, 08.12.2021 01:00



English, 08.12.2021 01:00



English, 08.12.2021 01:00



English, 08.12.2021 01:00

Mathematics, 08.12.2021 01:00

Health, 08.12.2021 01:00

Mathematics, 08.12.2021 01:00