Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 09:10
How have the greenhouse gasses increased from the year 2000 to 2018
Answers: 2

Chemistry, 22.06.2019 11:00
Surface currents are caused by blank space . question 14 options: surface currents are caused by? differences in water temperature high salinity differences in density wind forces
Answers: 1

Chemistry, 22.06.2019 12:00
1. if you have a gas at 127 degrees c, what is it's absolute temperature (kelvin)? a. 200kb. 300kc. 400kd. 500k2. if you had a gas whose absolute temperature measured 45 k, what is that temperature in celsius? a. -228 cb. -300 cc. 125 cd. 112 c
Answers: 2

Chemistry, 22.06.2019 19:00
A4.86 g piece of metal was placed in a graduated cylinder containing 15.5 ml of water. the water level rose to 17.3 ml. what is the density of the metal. i need the steps of how to solve it to so i can use a formula to work out other problems.
Answers: 1
You know the right answer?
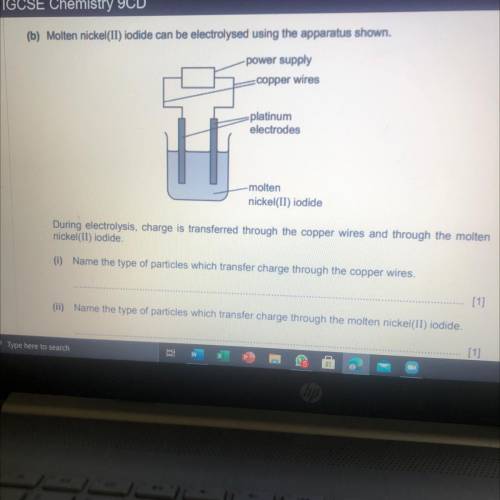
Predict the products of the electrolysis of molten nickel(II) iodide. Write an ionic half-equation f...
Questions

Computers and Technology, 05.01.2022 17:30


Mathematics, 05.01.2022 17:30

Social Studies, 05.01.2022 17:30

Mathematics, 05.01.2022 17:30

Social Studies, 05.01.2022 17:40






Social Studies, 05.01.2022 17:40


SAT, 05.01.2022 17:40


Business, 05.01.2022 17:40




Business, 05.01.2022 17:40