
Chemistry, 04.03.2021 07:10 sierram298
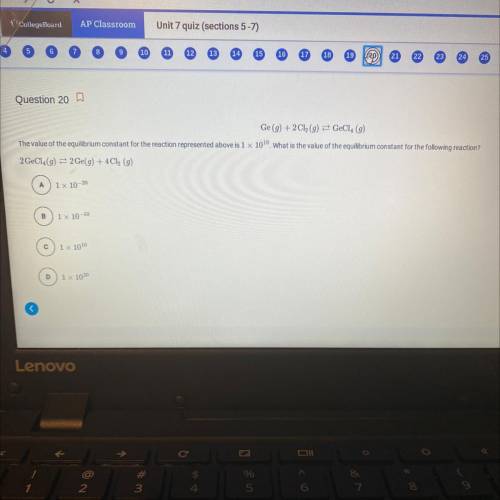
Ge (g) + 2Cl(g) = GeCl4 (g)
The value of the equilibrium constant for the reaction represented above is 1 x 100. What is the value of the equilibrium constant for the following reaction?
2 GeCl (g) = 2 Ge(g) +4Cl (g)
a) 1x 10-20
b) 1x 10-10
c) 1x 1010
d) 1 x 1020
will give brainliest !!

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 21:30
An alcohol thermometer makes use of alcohol's changing in order to measure temperature. as the temperature goes up, the alcohol contained in the thermometer increases in volume, filling more of the thermometer's tube.
Answers: 3


Chemistry, 22.06.2019 16:10
Amixture initially contains a, b, and c in the following concentrations: [a] = 0.300 m , [b] = 1.05 m , and [c] = 0.550 m . the following reaction occurs and equilibrium is established: a+2b⇌c at equilibrium, [a] = 0.140 m and [c] = 0.710 m . calculate the value of the equilibrium constant, kc.
Answers: 1

Chemistry, 23.06.2019 05:30
Idont understand it 1.what is the boiling point of a solution of 675 grams of ethylene glycol (c2h6o2) in 2.50 liters of water?
Answers: 2
You know the right answer?
Ge (g) + 2Cl(g) = GeCl4 (g)
The value of the equilibrium constant for the reaction represented abov...
Questions




English, 13.09.2021 19:40

Mathematics, 13.09.2021 19:40

Arts, 13.09.2021 19:40




Mathematics, 13.09.2021 19:40

Computers and Technology, 13.09.2021 19:40

English, 13.09.2021 19:40

History, 13.09.2021 19:40

Physics, 13.09.2021 19:40

Mathematics, 13.09.2021 19:40



Mathematics, 13.09.2021 19:40





