
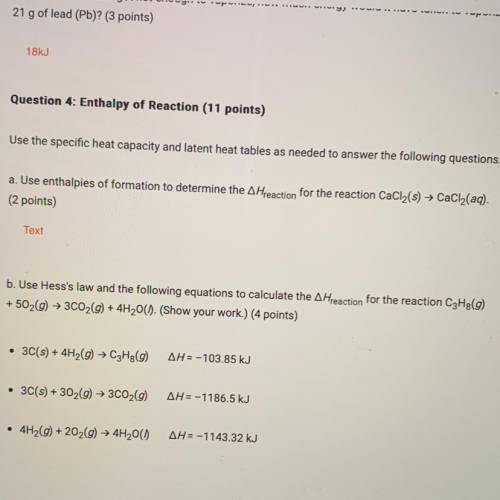
B. Use Hess's law and the following equations to calculate the A Hreaction for the reaction C3H8(9)
+ 502(9) + 3C02(9) + 4H2O(). (Show your work.) (4 points)
• 3C(s) + 4H2(9) → C3Hg(9)
AH = -103.85 kJ
• 3C(s) + 302(g) + 3C02(9)
AH=-1186.5 kJ
• 4H2(9) +202(9) + 4H2O()
AH=-1143.32 kJ

Answers: 3


Another question on Chemistry



Chemistry, 22.06.2019 02:30
Which piece of equipment would me most useful for measuring the volume of some water? a. pan balance b. graduated cylinder c. tweezers d. flask quick
Answers: 2

Chemistry, 22.06.2019 05:30
What is the mass defect of a mole of nuclei with 1.8 x 10^15 j/mol binding energy?
Answers: 1
You know the right answer?
B. Use Hess's law and the following equations to calculate the A Hreaction for the reaction C3H8(9)...
Questions


Mathematics, 05.05.2020 10:51






English, 05.05.2020 10:51

Mathematics, 05.05.2020 10:51

English, 05.05.2020 10:51


Biology, 05.05.2020 10:51

Social Studies, 05.05.2020 10:51



Mathematics, 05.05.2020 10:51




Chemistry, 05.05.2020 10:51



