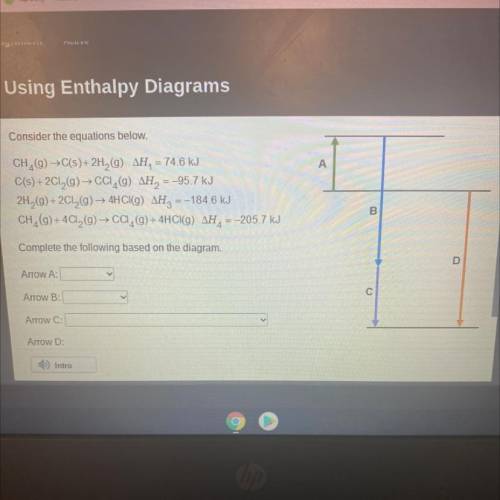
Consider the equations below.
CH,(9) →C(s)+2H2 (9) AH, = 74.6 kJ
C(s) + 2Cl2(g) → CCI (9) AH2...

Chemistry, 03.03.2021 01:10 angelashaw449
Consider the equations below.
CH,(9) →C(s)+2H2 (9) AH, = 74.6 kJ
C(s) + 2Cl2(g) → CCI (9) AH2 = -95.7 kJ
2H2(g) + 2Cl2(g) → 4HCI(9) AH2 = -184.6 kJ
CH, (g) + 4C12(g) → CCI,(9) + 4HCl(g) AH, = -205.7 kJ
Complete the following based on the diagram.
Arrow A:
Arrow B:
Arrow C:
Arrow D:

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 12:30
Achemist determined by measurements that 0.0300 most of beryllium oxide participate in a chemical reaction calculate the mass of berlylium oxide that participates
Answers: 3

Chemistry, 21.06.2019 22:00
Fission of uranium-235 products energy and a. isotopes of smaller elements b. isotopes of larger elements c. lighter isotopes of uranium d. heavier isotopes of uranium
Answers: 3

Chemistry, 22.06.2019 05:30
Which other elements contain the same number of outer electrons as sodium
Answers: 3

You know the right answer?
Questions






















