
Chemistry, 02.03.2021 14:00 camiserjai1832
Ok we trying this again. so just in case if the picture isnt showing, here are the questions:
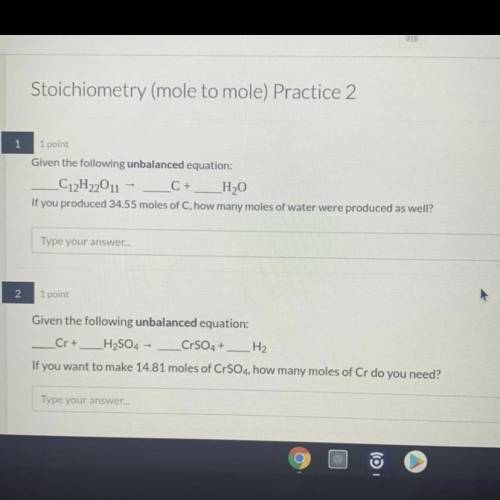
1. Given the unbalanced equation:
C12H22O11 = C + H2O
If you produced 34.55 moles of C, how many moles of water were produced as well?
2. Given the unbalanced equation:
Cr + H2SO4 = CrSO4 + H2
If you want to make 14.81 moles of CrSO4, how many moles of Cr do you need?

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 00:30
In numbering carbon atoms in the parent chain of a hydrocarbon, why would you number from right to left, rather than left to right
Answers: 1

Chemistry, 22.06.2019 04:00
What layer of the atmosphere is directly above the troposphere?
Answers: 1

Chemistry, 22.06.2019 06:00
How many atoms of mg are present in 97.22 grams of mg? 6.022 × 1023 2.408 × 1024 4.818 × 1024 5.855 × 1025
Answers: 2

Chemistry, 22.06.2019 06:40
Which statement is usually true about the relationship between activation energy and reaction rates? low activation energy barriers result in low rates. high activation energy barriers result in low rates. low activation energy barriers result in no reaction. high activation energy barriers result in no reaction.
Answers: 3
You know the right answer?
Ok we trying this again. so just in case if the picture isnt showing, here are the questions:
1. Gi...
Questions



Mathematics, 01.03.2021 22:10





Computers and Technology, 01.03.2021 22:10


Mathematics, 01.03.2021 22:10


Mathematics, 01.03.2021 22:10



Mathematics, 01.03.2021 22:10

Mathematics, 01.03.2021 22:10

English, 01.03.2021 22:10


Biology, 01.03.2021 22:10



