
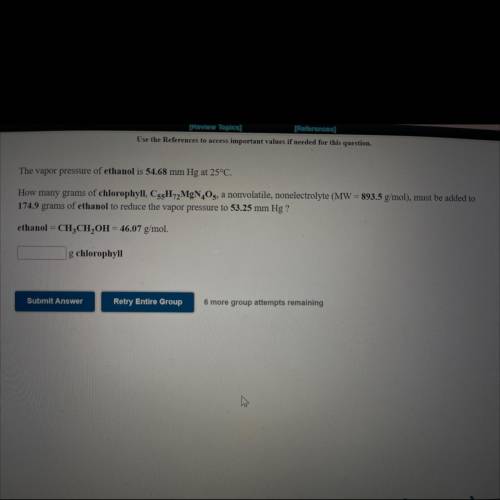
The vapor pressure of ethanol is 54.68 mm Hg at 25°C.
How many grams of chlorophyll, C55H72MgN4O5, a nonvolatile, nonelectrolyte (MW = 893.5 g/mol), must be added to
174.9 grams of ethanol to reduce the vapor pressure to 53.25 mm Hg ?
ethanol = CH3CH2OH = 46.07 g/mol.
How many ___g chlorophyll?

Answers: 3


Another question on Chemistry



Chemistry, 22.06.2019 09:00
What type of energy do chemical bonds have? what type of energy is it converted to during chemical reactions? question 15 options: chemical bonds have kinetic energy, which is converted to potential energy during chemical reactions. chemical bonds have electric energy, which is converted to potential energy during chemical reactions. chemical bonds have heat energy, which is converted to kinetic energy during chemical reactions. chemical bonds have potential energy, which is converted to heat energy during chemical reactions.
Answers: 1

Chemistry, 22.06.2019 10:30
If you add 5.00 ml of 0.100 m sodium hydroxide to 50.0 ml of acetate buffer that is 0.100 m in both acetic acid and sodium acetate, what is the ph of the resulting solution? acetic acid: ka = 1.8. x 10-5
Answers: 1
You know the right answer?
The vapor pressure of ethanol is 54.68 mm Hg at 25°C.
How many grams of chlorophyll, C55H72MgN4O5,...
Questions

Mathematics, 22.09.2019 04:30



Mathematics, 22.09.2019 04:30

Physics, 22.09.2019 04:30



History, 22.09.2019 04:30




English, 22.09.2019 04:30


Physics, 22.09.2019 04:30


Mathematics, 22.09.2019 04:30


Mathematics, 22.09.2019 04:30


Physics, 22.09.2019 04:30



