

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 16:00
Use the table to identify the phase and phase changes of the elements under the given conditions. write the name of the substance, phase, or phase change. when the temperature in a room increases from 25°c to 33°c, changes from a solid to a liquid. in a lab, methane and nitrogen are cooled from -170°c to -200°c. the methane freezes and the nitrogen . when gold is heated to 2,856°c it changes from a liquid to a .
Answers: 2

Chemistry, 22.06.2019 00:30
You have 125g of a certain seasoning and are told that it contains 76.0 g of salt what is the percentage of salt by mass in this seasoning
Answers: 1


Chemistry, 22.06.2019 12:40
In the following table, all the columns for the element calcium are filled out correctly. element electron structure of atom electron structure of ion net ionic charge calcium 1s22s22p63s23p64s2 1s32s22p63s23p64s1 +1 true false
Answers: 2
You know the right answer?
NaCl is purified by adding HCl to a saturated solution of NaCl (317 g/L). Will pure NaCl precipitate...
Questions

Mathematics, 16.01.2021 04:10





Computers and Technology, 16.01.2021 04:10

Social Studies, 16.01.2021 04:10

Chemistry, 16.01.2021 04:10



Biology, 16.01.2021 04:10




History, 16.01.2021 04:10

Arts, 16.01.2021 04:10

Health, 16.01.2021 04:10


History, 16.01.2021 04:10

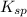 of the NaCl and according to Le C h a t e l i e r's principle, NaCl will precipitate out of the solution
of the NaCl and according to Le C h a t e l i e r's principle, NaCl will precipitate out of the solution

