Chemistry, 02.03.2021 03:40 makailaaa2
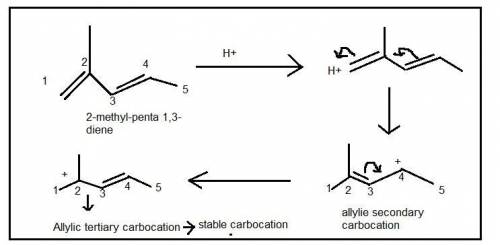
5. Write the two resonance hybrids for the carbocation that would be formed by protonation at C-1 of 2-methyl-1,3-pentadiene. Without doing a calculation, would you expect C-2 or C-4 (the two end carbons of the allylic cation) to have the most positive charge on it

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 15:30
Using the first volume and temperature reading on the table as v1 and t1, solve for the unknown values in the table below. remember to use the rules of significant figures when entering your numeric response.
Answers: 2

Chemistry, 22.06.2019 20:20
Nitric acid can be formed in two steps from the atmospheric gases nitrogen and oxygen, plus hydrogen prepared by reforming natural gas. in the first step, nitrogen and hydrogen react to form ammonia: (g) (g) (g) in the second step, ammonia and oxygen react to form nitric acid and water: (g) (g) (g) (g) calculate the net change in enthalpy for the formation of one mole of nitric acid from nitrogen, hydrogen and oxygen from these reactions. round your answer to the nearest .
Answers: 3

Chemistry, 23.06.2019 06:30
The molar mass of cu is 63.55 g/mol. the number of grams of cu produced in this reaction is
Answers: 3

Chemistry, 23.06.2019 13:30
Determine the osmotic pressure at 25 °c of an aqueous solution that is 0.028 m nano3. a) 0.685 atm b) 0.0729 atm c) 1.37 atm d) 0.0364 atm e) 2.06 atm
Answers: 2
You know the right answer?
5. Write the two resonance hybrids for the carbocation that would be formed by protonation at C-1 of...
Questions

Mathematics, 01.04.2021 19:10

History, 01.04.2021 19:10

English, 01.04.2021 19:10

Mathematics, 01.04.2021 19:10

Mathematics, 01.04.2021 19:10


Computers and Technology, 01.04.2021 19:10

Mathematics, 01.04.2021 19:10

Mathematics, 01.04.2021 19:10


Mathematics, 01.04.2021 19:10


Mathematics, 01.04.2021 19:10

Geography, 01.04.2021 19:10

Chemistry, 01.04.2021 19:10



Mathematics, 01.04.2021 19:10

Mathematics, 01.04.2021 19:10