
Chemistry, 02.03.2021 01:00 smartcookie8251
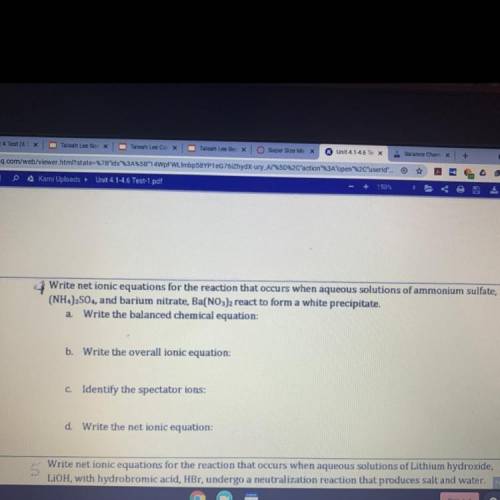
Write net ionic equations for the reaction that occurs when aqueous solutions of ammonium sulfate
(NH4)2SO,, and barium nitrate, Ba(NO3)2 react to form a white precipitate.
a Write the balanced chemical equation:
b. Write the overall ionic equation:
C. Identify the spectator ions:
d Write the net ionic equation:

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 06:30
Use examples from the article to explain one positive and one negative effect that chemistry has had on society.
Answers: 2

Chemistry, 22.06.2019 14:20
Which of the following are sources of revenue for media companies? a. direct sales to producers b.advertising and subscriptions c. online purchase d. capital investments
Answers: 1

Chemistry, 22.06.2019 18:30
Read the claim. breakfast is an important meal. it jump starts the body’s process of using calories to break down food. appetite can decrease with age, but going too long without eating causes metabolism to slow down. current research shows that incorporating legumes such as lentils and chickpeas into meals boosts metabolism for twenty-four hours. who might benefit from this claim? people who have a fast metabolism stores that sell exercise equipment people who take vitamin supplements grocery stores that sell legumes
Answers: 1

Chemistry, 23.06.2019 04:00
How much energy is required to vaporize 2 kg of copper? a 4730 kj b 207kj c 9460 kj d 414kj
Answers: 1
You know the right answer?
Write net ionic equations for the reaction that occurs when aqueous solutions of ammonium sulfate
(...
Questions

Business, 23.07.2019 15:50

Social Studies, 23.07.2019 15:50

Mathematics, 23.07.2019 15:50

Biology, 23.07.2019 15:50

Business, 23.07.2019 15:50

Business, 23.07.2019 15:50

Chemistry, 23.07.2019 15:50

Business, 23.07.2019 15:50

Social Studies, 23.07.2019 15:50

Business, 23.07.2019 15:50

Social Studies, 23.07.2019 15:50







Mathematics, 23.07.2019 15:50




