Chemistry, 01.03.2021 23:20 junior2461
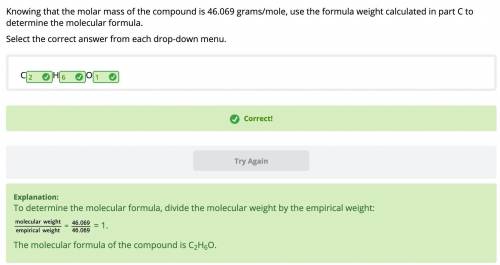
Knowing that the molar mass of the compound is 46.069 grams/mole, use the formula weight calculated in part C to
determine the molecular formula.
Select the correct answer from each drop-down menu.
С_H_O_

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 03:30
Explain why pure hydrogen cyanide does not conduct electricity, but become a conductor when it is dissolved in water? (at room temp, pure hcn exists as a volatile liquid)
Answers: 1

Chemistry, 22.06.2019 08:00
Identify a strong intermolecular force of attraction between an alcohol
Answers: 1

Chemistry, 22.06.2019 09:00
Astudent is asked to identify and element that is pale yellow brittle solid and does not conduct electricity. at which location in this periodic table would the element most likely be found?
Answers: 2

Chemistry, 22.06.2019 15:00
Areaction is first order with respect to reactant x and second order with respect to reactant y. which statement describes the rate law for this reaction?
Answers: 1
You know the right answer?
Knowing that the molar mass of the compound is 46.069 grams/mole, use the formula weight calculated...
Questions


English, 12.03.2021 21:00

Social Studies, 12.03.2021 21:00

Mathematics, 12.03.2021 21:00

Social Studies, 12.03.2021 21:00



Mathematics, 12.03.2021 21:00


English, 12.03.2021 21:00



Mathematics, 12.03.2021 21:00


Mathematics, 12.03.2021 21:00


Mathematics, 12.03.2021 21:00


English, 12.03.2021 21:00