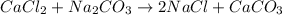Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 22:30
Determine the wavelength of the light absorbed when an electron in a hydrogen atom makes a transition from an orbital in the n=3 level to an orbital in the n=7 level.
Answers: 2

Chemistry, 22.06.2019 10:00
Which sentence about particles in matter is true? a. atoms are present in solids and liquids but not in gases. b. the particles of matter are in constant motion. c. the same kinds of atoms are found in different elements. d. when a solid changes to a liquid, the sizes of the particles change.
Answers: 1

Chemistry, 22.06.2019 23:00
What is formed when amino acids form long chains or polymerize
Answers: 1

Chemistry, 23.06.2019 03:00
What volume does 1.70 ×10–3 mol of chlorine gas occupy if its temperature is 20.2 °c and its pressure is 795 mm hg?
Answers: 3
You know the right answer?
Calculate the volume in mL of 0.100 of CaCl2 needed to produce 1.00 g of CaCO3. there is an excess o...
Questions

History, 27.01.2021 19:50


Mathematics, 27.01.2021 19:50

Mathematics, 27.01.2021 19:50



Biology, 27.01.2021 19:50


Mathematics, 27.01.2021 19:50

World Languages, 27.01.2021 19:50


Arts, 27.01.2021 19:50


Physics, 27.01.2021 19:50




English, 27.01.2021 19:50


Mathematics, 27.01.2021 19:50