
Chemistry, 01.03.2021 22:20 jacobp0712
In the lab activity, the reaction rate was determined by the appearance of a product. However, the reaction rate can also be determined by the disappearance of a reactant. Rate =Δ[product]/Δt or rate−Δ[reactant]Δ t. In each situation below, you are given a rate measured by the appearance of one component of the reaction and are asked to predict the rate of appearance or disappearance of another component, based on logic and stoichiometric relationships.
For example, if the reaction is as follows:
A+2B⟶products
For every mole of A that is used, 2 moles of B are used so the rate of disappearance of B is twice the rate of the disappearance of A.
This may be expressed as:
rate=−Δ[B]/Δt=−2[A]/Δt , N2(g)+3H2(g)⟶2NH3(g)
The reaction rate is measured as 0.032 M NH3/s. Determine the rate of disappearance of N2 and the rate of disappearance of H2. Explain how you arrived at your answers.

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 23:00
Matches the chemical name of each oxide of phosphorus to its chemical formula
Answers: 2

Chemistry, 22.06.2019 09:00
At 300 mm hg, a gas has a volume of 380 l, what is the volume at standard pressure
Answers: 1


Chemistry, 22.06.2019 11:00
What is the temperature of 0.750 mol of a gas stored in a 6,850 ml cylinder at 2.21 atm? . 2.95 k 5.24 k 138 k 246 k
Answers: 3
You know the right answer?
In the lab activity, the reaction rate was determined by the appearance of a product. However, the r...
Questions


History, 09.09.2021 01:50

Mathematics, 09.09.2021 01:50

Social Studies, 09.09.2021 01:50

Biology, 09.09.2021 01:50


Mathematics, 09.09.2021 01:50



Mathematics, 09.09.2021 01:50


Mathematics, 09.09.2021 01:50

Mathematics, 09.09.2021 01:50

Social Studies, 09.09.2021 01:50



Mathematics, 09.09.2021 01:50

Mathematics, 09.09.2021 01:50


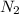 = 0.032 M/s
= 0.032 M/s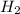 = 0.096 M/s
= 0.096 M/s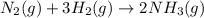
![-\frac{1d[N_2]}{dt}](/tpl/images/1158/2044/f2cf6.png)
![-\frac{1d[H_2]}{3dt}](/tpl/images/1158/2044/96c4e.png)
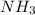 =
= ![\frac{1d[NH_3]}{2dt}](/tpl/images/1158/2044/ceb35.png)
![-\frac{1d[N_2]}{dt}=-\frac{1d[H_2]}{3dt}=\frac{1d[NH_3]}{2dt}](/tpl/images/1158/2044/4e2ff.png)
![-\frac{d[H_2]}{dt}=3\times 0.032M/s=0.096M/s](/tpl/images/1158/2044/7af2b.png)


