Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 00:10
Select the correct answer. which phrase correctly describes temperature? o a. average rotational kinetic energy of the particles in an object o b. average energy of the particles in an object c. average translational kinetic energy of the particles in an object od. all energy possessed by the particles in an object
Answers: 1



Chemistry, 23.06.2019 03:00
What do electromagnetic waves carry? how are they produced through which media can they move? where do they transfer energy? what do they not transfer? what do mechanical waves carry? how are they produced? through which media can they move? where do they transfer energy? what do they not transfer?
Answers: 1
You know the right answer?
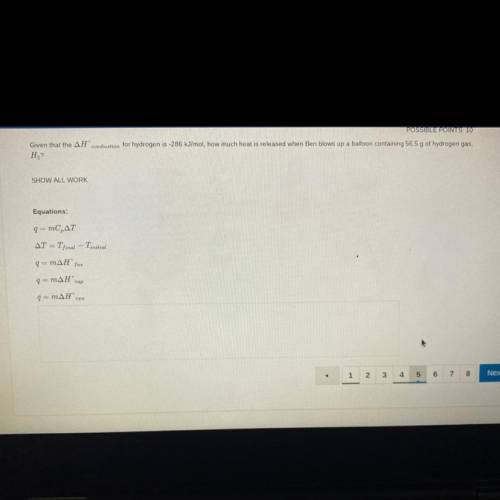
Give that the combustion for hydrogen is -286kJ/mol, how much heat is released when Ben blows up a b...
Questions



Mathematics, 18.03.2021 01:50





Mathematics, 18.03.2021 01:50

History, 18.03.2021 01:50




Mathematics, 18.03.2021 01:50

Chemistry, 18.03.2021 01:50




Mathematics, 18.03.2021 01:50

Mathematics, 18.03.2021 01:50