
Chemistry, 01.03.2021 21:50 lanaiheart7
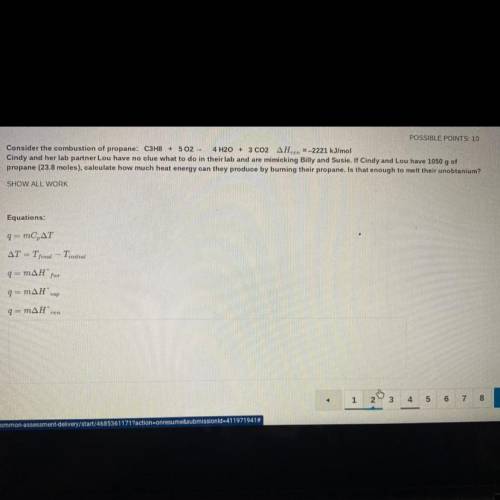
Consider the combustion of propane: C3H8 + 502 - 4 H2O + 3 CO2 AHzn =-2221 kJ/mol
Cindy and her lab partner Lou have no clue what to do in their lab and are mimicking Billy and Susie. If Cindy and Lou have 1050 g of
propane (23.8 moles), calculate how much heat energy can they produce by burning their propane. Is that enough to melt their unobtanium?

Answers: 2


Another question on Chemistry


Chemistry, 22.06.2019 12:10
Building glycogen from glucose molecules is an example of
Answers: 3

Chemistry, 22.06.2019 14:50
Which of the following is most likely true about water in chemical solutions?
Answers: 1

Chemistry, 22.06.2019 20:30
Select all the correct answers.which compounds have the empirical formula ch20? (multiple answers)a.c2h4o2b.c3h603c.ch2o2d.c5h1005e.c6h1206
Answers: 2
You know the right answer?
Consider the combustion of propane: C3H8 + 502 - 4 H2O + 3 CO2 AHzn =-2221 kJ/mol
Cindy and her lab...
Questions


Social Studies, 24.01.2021 14:00

Social Studies, 24.01.2021 14:00

Mathematics, 24.01.2021 14:00

Arts, 24.01.2021 14:00

English, 24.01.2021 14:00




Chemistry, 24.01.2021 14:00




English, 24.01.2021 14:00

English, 24.01.2021 14:00



Business, 24.01.2021 14:00

Mathematics, 24.01.2021 14:00

World Languages, 24.01.2021 14:00



