
Chemistry, 01.03.2021 18:50 emmanuelmashao6704
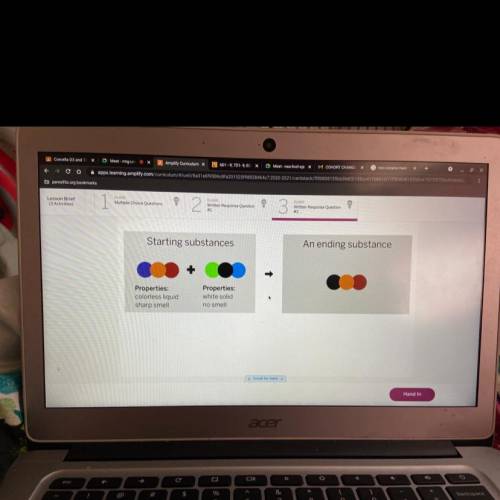
A chemist mixed two substances together: a colorless liquid with a strong smell and a white solid with no smell. The
substances' repeating groups of atoms are shown above on the left. After they were mixed, the chemist analyzed the
results and found two substances. One ending substance had the repeating group of atoms shown above on the right.
Is the ending substance the same substance as the colorless liquid? What happened to the atoms of the starting
substances when the ending substances formed? Be sure to explain your answers to both of these questions.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 04:00
Write the balanced equation for a reaction between aqueous nitric acid (hno3) and solid lithium metal (this is a single replacement reaction)
Answers: 1

Chemistry, 22.06.2019 04:40
*will mark you brainliest + 15 points ** why does the equilibrium of a system shift when the pressure is increased? a. to maximize the stress on the system b. to stop restoring equilibrium to the system c. to increase the total moles of gas in the system d. to decrease the total moles of gas in the system
Answers: 3

Chemistry, 22.06.2019 11:30
If blood contains 150g of hemoglobin per liter of blood, how much hemoglobin would be contained in 10 ml of blood
Answers: 2

Chemistry, 22.06.2019 15:00
Many ionic compounds and a few highly polar covalent compounds are because they completely ionize in water to create a solution filled with charged ions that can conduct an electric current.
Answers: 1
You know the right answer?
A chemist mixed two substances together: a colorless liquid with a strong smell and a white solid wi...
Questions



Social Studies, 11.10.2019 05:30


Biology, 11.10.2019 05:30



Biology, 11.10.2019 05:30



Biology, 11.10.2019 05:30

Mathematics, 11.10.2019 05:30

Mathematics, 11.10.2019 05:30

History, 11.10.2019 05:30


Mathematics, 11.10.2019 05:30

Health, 11.10.2019 05:30

Biology, 11.10.2019 05:30




