
Chemistry, 27.02.2021 14:10 kris22elizondop9v1bb
Calculate the PH of a 0.002M acetic acid solution if it is 2.3% ionised at this dilution ka=1.8×10⁸
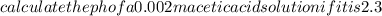

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 01:30
Pls! plant cells and animal cells were observed under a microscope. the characteristics of two cells are listed below. cell p: does not capture sunlight cell q: has cytoplasm but no chloroplast which statement about the two cells is correct? cell q also has a cell wall. cell q also has large vacuole. cell p also has a large vacuole. cell p also has a cell membrane.
Answers: 1

Chemistry, 22.06.2019 18:50
Question 3(multiple choice worth 4 points) (04.04 lc) what does it mean when an element is reduced? it empties a valance shell, reducing its atomic radius. it gains electrons, reducing its overall charge. it increases electronegativity, reducing its ability to bond. it loses electrons, reducing its electron number.
Answers: 1


Chemistry, 23.06.2019 00:00
What conclusion can you draw from this experiment about the components of the black ink?
Answers: 3
You know the right answer?
Calculate the PH of a 0.002M acetic acid solution if it is 2.3% ionised at this dilution ka=1.8×10⁸...
Questions


World Languages, 27.03.2021 03:30


Engineering, 27.03.2021 03:30


English, 27.03.2021 03:30



Mathematics, 27.03.2021 03:30

History, 27.03.2021 03:30


Spanish, 27.03.2021 03:30


Mathematics, 27.03.2021 03:30


Mathematics, 27.03.2021 03:30


Mathematics, 27.03.2021 03:30

Arts, 27.03.2021 03:30

Mathematics, 27.03.2021 03:30



