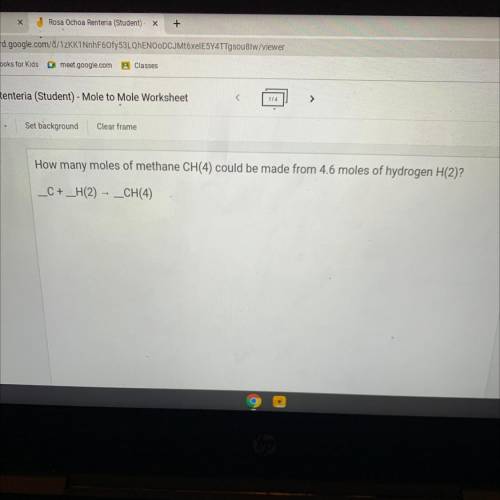
How many moles of methane CH(4) could be made from 4.6 moles of hydrogen H(2)?
_C+ _H(2) - CH(...

Chemistry, 27.02.2021 14:00 adejumoayobami1
How many moles of methane CH(4) could be made from 4.6 moles of hydrogen H(2)?
_C+ _H(2) - CH(4)

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 17:30
The following reaction shows the products when sulfuric acid and aluminum hydroxide react. al(oh)3 + h2so4 → al2(so4)3 + h2o the table shows the calculated amounts of reactants and products when the reaction was conducted in a laboratory. initial mass and yield sulfuric acid aluminum hydroxide initial amount of reactant 40 g 15 g theoretical yield of water from reactant 14.69 g 10.38 g what is the approximate amount of the leftover reactant? 11.73 g of sulfuric acid 10.33 g of sulfuric acid 11.12 g of aluminum hydroxide 13.67 g of aluminum hydroxide
Answers: 1

Chemistry, 22.06.2019 11:00
Which element would mostly likely have an electron affinity measuring closest to zero
Answers: 3

Chemistry, 22.06.2019 12:30
A50.0 ml sample of gas at 20.0 atm of pressure is compressed to 40.0 atm of pressure at constant temperature. what is the new volume? 0.0100 ml 0.325 ml 25.0 ml 100. ml
Answers: 1

You know the right answer?
Questions





Chemistry, 21.04.2020 01:58

Mathematics, 21.04.2020 01:58

Mathematics, 21.04.2020 01:58


Mathematics, 21.04.2020 01:58

History, 21.04.2020 01:58



Mathematics, 21.04.2020 01:58


History, 21.04.2020 01:58

Mathematics, 21.04.2020 01:58


Mathematics, 21.04.2020 01:58

Mathematics, 21.04.2020 01:58



