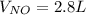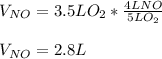Chemistry, 27.02.2021 08:00 georgesarkes12
4NH3(g) + 5O2(g) -> 4NO(g) +6H20(g) If 3.5 L of oxygen gas at STP react with an excess amount of ammonia, how many liters of nitrogen monoxide will be produced

Answers: 2


Another question on Chemistry


Chemistry, 22.06.2019 12:50
What is the chemical name of the compound na2co3? use the list of polyatomic ions and the periodic table to you answer. a. sodium carbon oxide b. sodium carbonate c. sodium(ll) carbonate d. sodium oxalate
Answers: 1

Chemistry, 22.06.2019 13:30
In a ni-cd battery, a fully charged cell is composed of nickelic hydroxide. nickel is an element that has multiple oxidation states. assume the following proportions of the states: nickel charge proportions found 0 0.17 +2 0.3 +3 0.33 +4 0.5 (a) determine the mean of the nickel charge. enter the answer to 2 decimal places.(b) determine the cumulative distribution function of nickel charge.
Answers: 2

Chemistry, 22.06.2019 15:00
Answer explain why it is not possible to deduce a complete order of reactivity.
Answers: 3
You know the right answer?
4NH3(g) + 5O2(g) -> 4NO(g) +6H20(g) If 3.5 L of oxygen gas at STP react with an excess amount of...
Questions

Chemistry, 26.03.2022 05:30






Physics, 26.03.2022 05:50


Mathematics, 26.03.2022 05:50

Mathematics, 26.03.2022 05:50



Mathematics, 26.03.2022 06:00

Mathematics, 26.03.2022 06:00


World Languages, 26.03.2022 06:00