

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 17:00
Why do sodium and neon have vastly different chemical and physical properties despite having similar atomic masses?
Answers: 2

Chemistry, 21.06.2019 22:00
If 1.63 times 10 negative 4 of helium dissolves in 100.0g of water, what is the concentration in parts per million
Answers: 3

Chemistry, 22.06.2019 08:30
For each of the compounds below, show that the charges on the ions add up to zero. a. kbr b. cao c. li(2)o d. cacl(2) e. alcl(3)
Answers: 2

Chemistry, 22.06.2019 08:30
If i initially have a gas at a pressure of 12 atm, a volume of 23 liters, and a temperature of 200 k, and then i raise the pressure to 14 atm and increase the temperature to 300 k, what is the new volume of the gas?
Answers: 1
You know the right answer?
Mg3(PO4)2+ 3K2SO4= 3MgSO4+ 2K3PO4. How many moles of potassium phosphate (K3PO4) will be produced fr...
Questions

English, 04.01.2021 21:10


Mathematics, 04.01.2021 21:10


Mathematics, 04.01.2021 21:10

Advanced Placement (AP), 04.01.2021 21:10

Mathematics, 04.01.2021 21:10


English, 04.01.2021 21:10


Mathematics, 04.01.2021 21:10

Mathematics, 04.01.2021 21:10

Mathematics, 04.01.2021 21:10


English, 04.01.2021 21:10

Mathematics, 04.01.2021 21:10


Mathematics, 04.01.2021 21:10


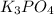 will be produced from 5.62 moles of magnesium phosphate
will be produced from 5.62 moles of magnesium phosphate 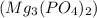
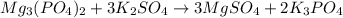
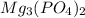 produce = 2 mole of
produce = 2 mole of 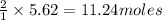 of
of 

