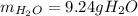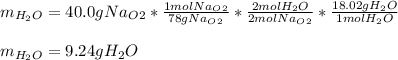A) What mass in grams of H20 is needed to react completely with 40.0 g of
Na2O2?
M(H2O)...

Chemistry, 26.02.2021 09:20 jagslovegirl
A) What mass in grams of H20 is needed to react completely with 40.0 g of
Na2O2?
M(H2O) =18.02g/mol
M(NA2O2)= 78g/mol
Ecuation:
2Na2O2 (s)+2h2O(I)—> 4NaOH(aq) + O2 (g)

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 00:30
This element exists in adundance in the sun.explain how you would go about capturing sunlight.would this captured sunlight contain any of the element?
Answers: 1

Chemistry, 22.06.2019 09:10
How have the greenhouse gasses increased from the year 2000 to 2018
Answers: 2

Chemistry, 22.06.2019 19:00
Which statement best describes what happens when molecular compounds melt
Answers: 1

Chemistry, 22.06.2019 19:30
Anurse used a 0.02-mg/l solution of disinfection to clean a patients wound. what is the concentration of the solution expressed as a percentage?
Answers: 1
You know the right answer?
Questions




Mathematics, 21.08.2020 01:01




Mathematics, 21.08.2020 01:01



English, 21.08.2020 01:01


Mathematics, 21.08.2020 01:01

Computers and Technology, 21.08.2020 01:01

Mathematics, 21.08.2020 01:01


Mathematics, 21.08.2020 01:01

Mathematics, 21.08.2020 01:01

Mathematics, 21.08.2020 01:01