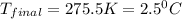Chemistry, 26.02.2021 06:20 princessss30188
A 100 mL sample of ethanol at 25°C is mixed with a 300 mL sample of ethanol at -5°C. The mixture is allowed to come to thermal equilibrium. What is the final temperature?

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 18:00
Which type of reaction always has an element and a compound as reactants
Answers: 1

Chemistry, 22.06.2019 05:50
What are transitions between a liquid and a solid called? identify which way they are transitioning
Answers: 2


Chemistry, 23.06.2019 04:00
Achemical reaction is done in the setup shown , resulting in a change of mass. what will happen if the same reaction is done in a sealed container that is placed on the electronic balance?
Answers: 2
You know the right answer?
A 100 mL sample of ethanol at 25°C is mixed with a 300 mL sample of ethanol at -5°C. The mixture is...
Questions




Geography, 04.10.2020 14:01



Mathematics, 04.10.2020 14:01





Mathematics, 04.10.2020 14:01


Mathematics, 04.10.2020 14:01




Mathematics, 04.10.2020 14:01


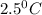
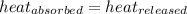
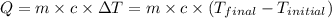
![m_1\times c_1\times (T_{final}-T_1)=-[m_2\times c_2\times (T_{final}-T_2)]](/tpl/images/1150/2880/09236.png) .................(1)
.................(1)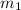 = mass of first sample of ethanol = 100 ml
= mass of first sample of ethanol = 100 ml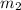 = mass of second sample of ethanol = 300 ml
= mass of second sample of ethanol = 300 ml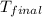 = final temperature = ?
= final temperature = ?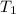 = temperature of first sample of ethanol =
= temperature of first sample of ethanol = 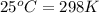
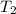 = temperature of second sample of ethanol =
= temperature of second sample of ethanol = 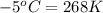
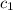 =
= 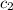 = specific heat of ethanol
= specific heat of ethanol![-100\times (T_{final}-298)=[300\times (T_{final}-268)]](/tpl/images/1150/2880/2eae3.png)