
Chemistry, 26.02.2021 04:40 gabrielleteti
How many moles of H3(PO4) are produced when 71.0 g P4O10 reacts
completely with H2O in the reaction?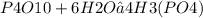

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 07:40
22. a flask containing 450 ml of 0.50 m h2so4 was accidentally knocked to the floor. how many grams of nahco, do you need to put on the spill to neutralize the acid according to the following equation: h2so4(aq)+2 nahcos(aq) na,so(aq) +2 h20()+2 co2(g) d) 38 g a) 2.3 g b) 9.5 g c) 19 g
Answers: 1

Chemistry, 23.06.2019 01:00
Chromium(iii) sulfate is a transition metal compound containing the metal chromium and the polyatomic ion sulfate. the oxidation state of chromium in this compound is , and the chemical formula of the compound is ( ) . reset next
Answers: 3

Chemistry, 23.06.2019 01:30
In which phase of mitosis do the spindle fibers pull the chromosomes apart to opposite sides of the cell ?
Answers: 1

Chemistry, 23.06.2019 02:30
What role does weathering have in shaping earth’s surface? a) it allows sediments to fall out of a medium. b) it sediments settle on a new surface. c) it breaks down older material into sediments. d) it transports sediments to a different location. will give brainliest, answer quickly.
Answers: 2
You know the right answer?
How many moles of H3(PO4) are produced when 71.0 g P4O10 reacts
completely with H2O in the reaction...
Questions




Mathematics, 21.04.2021 01:00

Mathematics, 21.04.2021 01:00





Social Studies, 21.04.2021 01:00


English, 21.04.2021 01:00

Mathematics, 21.04.2021 01:00


Biology, 21.04.2021 01:00

Biology, 21.04.2021 01:00






