
Chemistry, 25.02.2021 23:20 kharmaculpepper
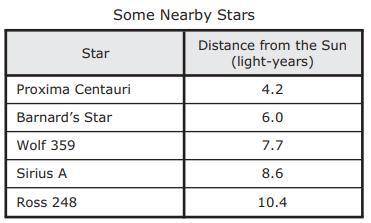
The table lists the distances of some of the nearest stars.
Based on the table, a student used a measuring tape to model the distances between the sun and these nearby stars. The sun was at the starting point. Proxima Centauri was at the 10 m mark.
Which star was closest to the 20 m mark in this model?
Sirius A
Ross 248
Barnard’s Star
Wolf 359

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 14:10
16. in a reaction that has reached equilibrium, a. the forward and reverse reactions are occurring at the same rate. b. the reactants and products are in equal concentrations. c. the forward reaction has gone further than the reverse reaction. d. there are equal numbers of atoms on both sides of the equation. e. a, b, and d are correct.
Answers: 2

Chemistry, 23.06.2019 10:10
Solid tin exists in two forms: white and gray. for the transformation sn(s, white) → sn(s, gray) the enthalpy change is -2.1 kj/mol and the entropy change is -7.4 j/(mol*k). a. calculate the gibbs free energy change for the conversion of 1.00 mol white tin to gray tin at -30℃. b. will white tin convert spontaneously to gray tin at -30℃? c. at what temperature are white and gray tin thermodynamically equivalent at a pressure of 1 atm?
Answers: 3

Chemistry, 23.06.2019 16:00
Be sure to answer all parts. the catalytic destruction of ozone occurs via a two-step mechanism, where x can be any of several species: (1) x + o3 → xo + o2 [slow] (2) xo + o → x + o2 [fast] (a) write the overall reaction. o3 + o → 2 o2 o3 + xo → x + 2 o2 xo + x + o3 + o → xo + x + 2 o2 x + o3 → xo + o2 (b) write the rate law for each step (using k for the rate constant). reaction 1: reaction 2: k[x][o2] k[xo][o2] k[x][o3] k[xo][o3] k[xo][o2] k[x][o] k[x][o2] k[xo][o] (c) x acts as a and xo acts as a catalyst intermediate intermediate catalyst (d) high-flying aircraft release no into the stratosphere, which catalyzes this process. when o3 and no concentrations are 3 ă— 1012 molecule/cm3 and 9.9 ă— 109 molecule/cm3, respectively, what is the rate of o3 depletion? the rate constant k for the rate-determining step is 6 ă— 10â’15 (cm3)2/moleculeâ·s. give your answer in scientific notation. use one significant figure in your answer. ă— 10 molecule/s
Answers: 2

Chemistry, 23.06.2019 21:00
Using the thermodynamic information in the aleks data tab, calculate the boiling point of titanium tetrachloride ticl4. round your answer to the nearest degree.
Answers: 1
You know the right answer?
The table lists the distances of some of the nearest stars.
Based on the table, a student used a me...
Questions


Mathematics, 04.08.2019 07:50




Biology, 04.08.2019 07:50


Biology, 04.08.2019 07:50



Biology, 04.08.2019 07:50

Mathematics, 04.08.2019 07:50

Spanish, 04.08.2019 07:50


Chemistry, 04.08.2019 07:50




History, 04.08.2019 07:50

Chemistry, 04.08.2019 07:50



