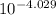Chemistry, 25.02.2021 21:10 lovelarissa
A buffer with a pH of 4.13 contains 0.29 M of sodium benzoate and 0.34 M of benzoic acid. What is the concentration of [H3O ] in the solution after the addition of 0.054 mol HCl to a final volume of 1.5 L

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 01:30
Phosphorous acid, h3po3(aq) , is a diprotic oxyacid that is an important compound in industry and agriculture. the values of phosphorous acid are 1.30 6.70 calculate the ph for each of the given points in the titration of 50.0 ml of 1.5 m h3po3(aq) with 1.5 m koh(aq) .
Answers: 3



Chemistry, 23.06.2019 11:00
Which of the following reactions is endothermic? h2(g) + ½ o2(g) h2o(g), h = -57.82 kcal ½n2(g) + o2(g) + 8.1 kcal no2(g) ½ n2(g) + 3/2 h2(g) nh3(g) + 11.0 kcal c(diamond) + o2(g) co2, h = -94.50 kcal
Answers: 2
You know the right answer?
A buffer with a pH of 4.13 contains 0.29 M of sodium benzoate and 0.34 M of benzoic acid. What is th...
Questions





Physics, 20.08.2019 22:40

Mathematics, 20.08.2019 22:40



History, 20.08.2019 22:40





Mathematics, 20.08.2019 22:40

World Languages, 20.08.2019 22:40

History, 20.08.2019 22:40

History, 20.08.2019 22:40

Mathematics, 20.08.2019 22:40

Social Studies, 20.08.2019 22:40