
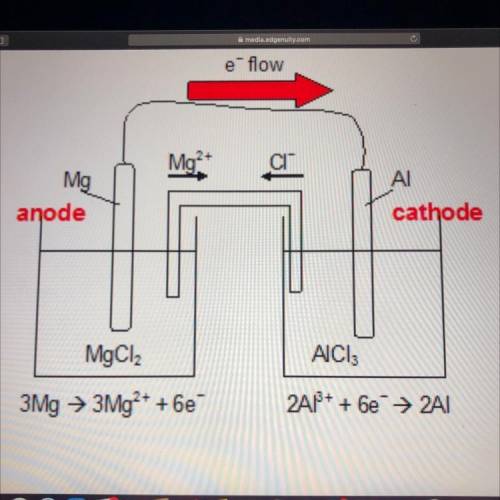
Look at the diagram of an electrochemical cell below.
Which observation would be most likely to happen if a power supply were added to the electrochemical cell?
A) Magnesium would be oxidized.
B) Electrons would flow through the salt bridge.
C) The AI electrode would become neutral.
D) The Mg electrode would become the cathode.

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 18:40
What is the binding energy of a nucleus that has a mass defect of 5.81*10-^29 kg a 5.23*10-^12 j b 3.15* 10^12 j c 1.57*10-3 j d 9.44*10^20 j
Answers: 1

Chemistry, 22.06.2019 20:30
Water undergoes a large change in density at 0 ∘ c as it freezes to form ice. calculate the percent change in density that occurs when liquid water freezes to ice at 0 ∘ c given that
Answers: 2

Chemistry, 23.06.2019 02:00
Which statement is true about the model of the electromagnetic spectrum a: it change the frequencies of light. b: it compare wavelengths of light. c: the color of light waves can be changed using the model. d: the intensities of light waves can be decreased using the model.
Answers: 2

Chemistry, 23.06.2019 03:50
What is the equation fort the alkaline zinc/manganese dioxide cell. a) anode b)cathode c)overall equations.
Answers: 2
You know the right answer?
Look at the diagram of an electrochemical cell below.
Which observation would be most likely to hap...
Questions





Computers and Technology, 13.12.2019 18:31

















