
Chemistry, 25.02.2021 18:40 fluffyanimal456
Copper reacts with silver nitrate to produce silver and copper(II) nitrate according to the equation below: Cu + 2AgNO3 →Cu(NO3)2 + 2Ag Calculate the number of moles of copper(II) nitrate produced when 5 moles of copper react. Type your answer as a number with 1 significant figure. Make sure to include the correct units in your answer. Units are a type of measurement i. e. gram (g) or mole (mol). Do not include the chemical formula in your answer.

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 23:00
At room temperature what happens to the average kinetic energy of the molecules of a solid, liquid, and a gas
Answers: 2

Chemistry, 22.06.2019 17:00
The biosphere of the earth is made up of what compound? organic or inorganic?
Answers: 3

Chemistry, 22.06.2019 21:40
A5 mole sample of liquid acetone is converted to a gas at 75.0°c. if 628 j are required to raise the temperature of the liquid to the boiling point, 15.600 kj are required to evaporate the liquid, and 712 j are required to raise the final temperature to 75.0°c, what is the total energy required for the conversion?
Answers: 3

Chemistry, 23.06.2019 06:00
What physical property of gold makes panning a useful way to get gold from streams?
Answers: 2
You know the right answer?
Copper reacts with silver nitrate to produce silver and copper(II) nitrate according to the equation...
Questions

Health, 05.01.2021 23:40


Physics, 05.01.2021 23:40



History, 05.01.2021 23:40

Mathematics, 05.01.2021 23:40

Mathematics, 05.01.2021 23:40

Mathematics, 05.01.2021 23:40

Biology, 05.01.2021 23:40

World Languages, 05.01.2021 23:40

Mathematics, 05.01.2021 23:40



Mathematics, 05.01.2021 23:40


Mathematics, 05.01.2021 23:40

Mathematics, 05.01.2021 23:40

Mathematics, 05.01.2021 23:40

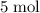 of
of 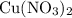 would be produced (assuming that reaction does not run out of
would be produced (assuming that reaction does not run out of 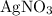 until all the
until all the 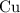 was converted.)
was converted.) 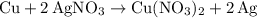 .
. . In other words, the actual equation for this reaction should be:
. In other words, the actual equation for this reaction should be: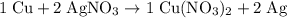 .
.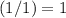 .
.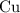 before running out of any other reactant.) This coefficient ratio would be equal to the ratio between:
before running out of any other reactant.) This coefficient ratio would be equal to the ratio between: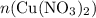 , the number of moles of
, the number of moles of 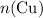 , the number of moles of
, the number of moles of 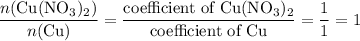 .
.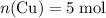 . Assume that
. Assume that 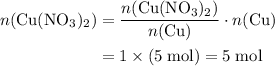 .
.

