
Chemistry, 25.02.2021 18:50 alexisbrad3107
Tris is a molecule that can be used to prepare buffers for biochemical experiments. It exists in two forms: Tris (a base) and TrisH (an acid). The MW of Tris base is 121.14 g/mol; the MW of TrisH is 157.6 g/mol (the extra weight is due to the Cl- counterion that is present in the acid). The Ka of the acid is 8.32 X 10-9. Assume that you have TrisH in solid form (a powder), unlimited 1M HCl, 1 M NaOH and distilled water. How would you prepare 1 L of a 0.02 M Tris Buffer, pH?

Answers: 3


Another question on Chemistry


Chemistry, 22.06.2019 06:00
Compare and contrast physical changes with chemical changes.
Answers: 1

Chemistry, 22.06.2019 14:30
Calculate the mass of carbon in 97.0 g of sucrose c12h22o11
Answers: 3

Chemistry, 22.06.2019 21:50
If e is the symbol for an element, which two of the following symbols represent isotopes of the same element? 1. e2. e3. ea.1 and 2c.1 and 4b.3 and 4d.2 and 3
Answers: 2
You know the right answer?
Tris is a molecule that can be used to prepare buffers for biochemical experiments. It exists in two...
Questions

Social Studies, 27.05.2020 18:59



Biology, 27.05.2020 18:59



Physics, 27.05.2020 18:59





Mathematics, 27.05.2020 18:59






History, 27.05.2020 18:59

History, 27.05.2020 18:59

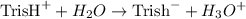
![$Ka = \frac{[\text{Tris}^- \times H_3O]}{\text{Tris}^+}$](/tpl/images/1147/5192/039c7.png)
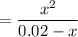
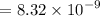
 , we have
, we have 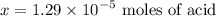
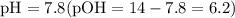 , one must have the
, one must have the 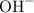 concentration of the :
concentration of the :![$\text{[OH}^-]=10^{-pOH} = 6.31 \times 10^{-7} \text{ moles of base}$](/tpl/images/1147/5192/2ecff.png)
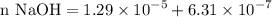
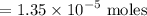
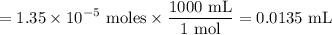
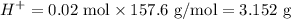
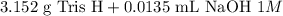 gauge to 1000 mL with water.
gauge to 1000 mL with water.

