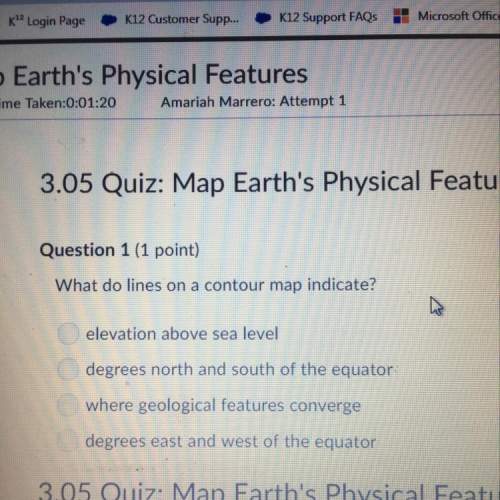
2Al2O3> 4A1+302
How many oxygen can be produced from 5 mol of aluminum oxide...

Chemistry, 25.02.2021 17:20 kitttimothy55
2Al2O3> 4A1+302
How many oxygen can be produced from 5 mol of aluminum oxide

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 20:20
Calculate the molarity of the solution. 6.02 x 1022 molecules of hci (molecular weight = 36.5 g/mole) in 2.0 liters of water m
Answers: 1


Chemistry, 22.06.2019 13:50
Read the chemical equation. 2c2h2 + 5o2 → 4co2 + 2h2o which of the following statements would be correct if one mole of c2h2 was used in this reaction? one mole of oxygen was used in this reaction. five moles of oxygen were used in this reaction. four moles of carbon dioxide were produced from this reaction. two moles of carbon dioxide were produced from this reaction.
Answers: 3

Chemistry, 22.06.2019 15:00
Large helium-filled balloons are used to lift scientific equipment to high altitudes. what is the pressure inside such a balloon if it starts out at sea level with a temperature of 10.0ºc and rises to an altitude where its volume is twenty times the original volume and its temperature is – 50.0ºc ?
Answers: 2
You know the right answer?
Questions






Mathematics, 26.08.2020 23:01








Computers and Technology, 26.08.2020 23:01


Mathematics, 26.08.2020 23:01


Mathematics, 26.08.2020 23:01


Physics, 26.08.2020 23:01