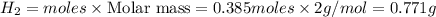Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 04:30
Electrons are extremely important to what area of technology? a) anti-aging research b) household product development c) electronics d) drug discovery
Answers: 3

Chemistry, 22.06.2019 04:40
Silver tarnishes as silver metal reacts with hydrogen sulfide, h2s, in the air. in this reaction, dark silver sulfide, au2s, covers the surface of silver. when silver is polished, this coating of silver sulfide can be removed from the surface. this makes the silver shiny again. enter the coefficients that balance the tarnishing reaction equation. (type 1 for no coefficient.)
Answers: 2

Chemistry, 22.06.2019 06:30
How many moles of carbon dioxide will form if 2.5 moles of c3h8 is burned
Answers: 1

You know the right answer?
‼️SHOW ALL STEPS‼️ If 14.4 grams of iron reacts with hydrochloric acid, how many grams of hydrogen g...
Questions



Mathematics, 19.02.2021 04:40


Mathematics, 19.02.2021 04:40



Mathematics, 19.02.2021 04:40

Chemistry, 19.02.2021 04:40

English, 19.02.2021 04:40

Mathematics, 19.02.2021 04:40



Computers and Technology, 19.02.2021 04:40

Mathematics, 19.02.2021 04:40

English, 19.02.2021 04:40


Mathematics, 19.02.2021 04:40


Biology, 19.02.2021 04:40

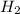 will be produced
will be produced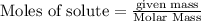
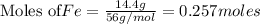
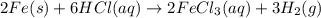
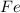 produce = 3 moles of
produce = 3 moles of 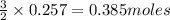 of
of