
Chemistry, 25.02.2021 01:00 marrissajade61191
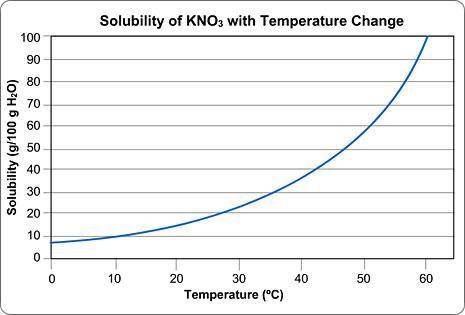
Imagine that you have 100g of water.
You start dissolving KNO3 in the water and you find that after you've dissolved about 55 g of KNO3, you can't dissolve any more; it just sinks to the bottom.
Approximately what is the temperature of the water?
Round your answer to the nearest whole number and submit the number only; no units.

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 23:00
What is the formula that this ionic compounds could form sr2+p3-o2-
Answers: 3

Chemistry, 22.06.2019 09:20
Sugar is dissolved in water. which is the solute? sugar neither both water
Answers: 1

Chemistry, 22.06.2019 19:20
Consider hydrogen in an excited state n = 5n=5 that emits photons to reach the ground state. there are various possible transitions other than straight to the ground state that can occur; for example, it can drop to the n = 3n=3 state followed by the n = 3n=3 to the ground state transition. which of the possible transitions will result in the emission of a photon in the visible region?
Answers: 3

Chemistry, 23.06.2019 01:30
What happens to the concentration of hydronium ions as the ph of a solution increases? a. hydronium ion concentration stays the same b. hydronium ion concentration decreases c. hydronium ion concentration increases
Answers: 1
You know the right answer?
Imagine that you have 100g of water.
You start dissolving KNO3 in the water and you find that after...
Questions



Physics, 11.09.2021 01:00

Mathematics, 11.09.2021 01:00

Mathematics, 11.09.2021 01:00



English, 11.09.2021 01:00



English, 11.09.2021 01:00




Mathematics, 11.09.2021 01:00

History, 11.09.2021 01:00

Mathematics, 11.09.2021 01:00

Mathematics, 11.09.2021 01:00


Mathematics, 11.09.2021 01:00



