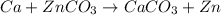Chemistry, 16.12.2019 05:31 xonyemaa12
Which best represents the reaction of calcium and zinc carbonate (znco3) to form calcium carbonate (caco3) and zinc?

Answers: 2


Another question on Chemistry

Chemistry, 23.06.2019 06:40
The combustion of methane, ch4, releases 890.4kj/mol. that is, when one mole of methane is burned,890.4 kj are given off to the surroundings. this meansthat the products have 890.4 kj less than the reactants.thus, ah for the reaction = - 890.4 kj. a negative symbolforah indicates an exothermic reaction.ch (g) + 20 (g)> co2 (g) + 2 h0 (1); ah = - 890.4 kga) how much energy is given off when 2.00 mol of ch,are burned? b) how much energy is released when 22.4g of ch. areburned?
Answers: 1

Chemistry, 23.06.2019 13:50
Which compounds are the brønsted-lowry bases in this equilibrium: hc2o4– + h2bo3– h3bo3 + c2o42– ? a. h2bo3– and h3bo3 b. hc2o4– and h3bo3 c. hc2o4– and c2o42– d. h2bo3– and c2o42– e. hc2o4– and h2bo3–
Answers: 2


Chemistry, 23.06.2019 22:00
The following compounds, listed with their boiling points, are liquid at –10ºc: butane, –0.5ºc; ethanol, 78.3ºc; toluene, 110.6ºc. at –10ºc, which of these liquids would you expect to have the highest vapor pressure? which the lowest? explain. (4 points)
Answers: 1
You know the right answer?
Which best represents the reaction of calcium and zinc carbonate (znco3) to form calcium carbonate (...
Questions

Mathematics, 03.02.2020 02:44

Chemistry, 03.02.2020 02:44


Chemistry, 03.02.2020 02:44



Social Studies, 03.02.2020 02:44





Biology, 03.02.2020 02:45

Mathematics, 03.02.2020 02:45

Mathematics, 03.02.2020 02:45

Mathematics, 03.02.2020 02:45

Mathematics, 03.02.2020 02:45

Mathematics, 03.02.2020 02:45



Mathematics, 03.02.2020 02:45